1. Context
Gastric ulcer disease, a prevalent and persistent gastrointestinal condition, poses a significant challenge to global health systems, particularly in low-income and middle-income countries, where its burden contributes substantially to premature mortality rates. Defined by erosions or defects within the mucosal lining of the stomach, gastric ulcers can lead to severe complications such as upper gastrointestinal (GI) bleeding, perforation, and, albeit less commonly, gastric outlet obstruction. These complications exact a heavy toll on affected individuals, leading to increased morbidity and mortality rates, thereby necessitating the development and implementation of effective preventive and therapeutic strategies (1, 2).
The etiology of gastric ulcers is multifaceted, with Helicobacter pylori infection and the use of non-steroidal anti-inflammatory drugs (NSAIDs), including aspirin, emerging as primary causative factors. Helicobacter pylori infection, a common bacterial infection affecting a significant portion of the global population, plays a central role in gastric ulcer pathogenesis by disrupting the delicate balance of gastric mucosal integrity (3). Concurrently, NSAIDs, widely utilized for their analgesic and anti-inflammatory properties, significantly elevate the risk of upper GI complications through the inhibition of mucosal prostaglandin production, impairing the stomach's ability to protect itself from injury (4).
To mitigate the risks associated with gastric ulcer disease, gastroprotectant drugs have been developed and extensively employed in clinical practice (5). These drugs, encompassing proton pump inhibitors (PPIs), prostaglandin analogues, and histamine-2 receptor antagonists (H2RAs), serve to safeguard the gastric mucosa, promote the healing of mucosal damage, and stabilize GI bleeding, thereby offering a multifaceted approach to gastric ulcer prevention and treatment. Among these agents, PPIs have emerged as the cornerstone of gastroprotective therapy, garnering support from systematic reviews and meta-analyses across various clinical settings (6).
However, despite the widespread use of gastroprotectant drugs, a comprehensive understanding of their effects across different clinical scenarios remains elusive. Existing literature highlights a notable gap in knowledge regarding the comparative effectiveness of these agents in diverse contexts, including prevention, healing, and acute bleeding episodes (7, 8). Moreover, the anatomical location of gastric ulcers, whether in the stomach or duodenum, may influence treatment outcomes and necessitate tailored therapeutic approaches.
In addition to addressing the efficacy of traditional gastroprotectant drugs such as PPIs, prostaglandin analogues, and H2RAs, our study aims to explore the emerging role of vonoprazan (VPZ) in gastric ulcer management. Recognizing VPZ's potent and enduring gastric acid suppression capabilities, as well as its potential to heal and prevent ulcers associated with NSAID and low-dose aspirin use, we seek to elucidate its comparative effectiveness against established therapies (9).
Given the evolving landscape of cardiovascular disease management and the widespread use of antithrombotic therapies, our study will investigate the implications of these therapies on gastric ulcer risk and management (10). With the advent of non-vitamin K antagonist direct oral anticoagulants (DOACs) and the complexities surrounding the discontinuation of antithrombotic therapy, particularly in patients undergoing dual antiplatelet therapy (DAPT), our analysis will provide insights into optimizing ulcer prevention strategies in this patient population. By synthesizing available data from a comprehensive literature search and conducting meta-analyses of RCTs, our study aims to inform evidence-based guidelines and optimize the use of gastroprotectant therapies in gastric ulcer prevention and management. Through meticulous analysis and rigorous assessment, we aspire to reduce the global burden of gastric ulcer disease and improve patient outcomes by providing clinicians with actionable recommendations tailored to diverse clinical scenarios and patient populations (11, 12).
Additionally, our study will delve into the mechanisms underlying corticosteroid-induced GI complications, a subject of ongoing debate since the 1950s. Despite advancements in medical research, the rarity of GI bleeding and perforation events has hampered efforts to conclusively ascertain the risk posed by corticosteroid therapy through traditional RCTs. Consequently, attention has turned towards observational studies as a means to explore these rare adverse effects effectively (13). However, the literature surrounding corticosteroid-induced GI complications reflects a landscape of uncertainty. In various databases and product monographs, descriptions of peptic ulcer disease and GI bleeding as potential adverse effects of corticosteroids remain inconsistent (14). Clinical recommendations mirror this ambiguity, with conflicting assertions regarding the ulcerogenic properties of corticosteroids and the necessity of antiulcer prophylaxis. Notably, recent surveys underscore the persisting perception among practitioners of corticosteroids as ulcerogenic agents, prompting a widespread inclination towards ulcer prophylaxis despite diverging clinical opinions (15).
The gravity of GI bleeding, peptic ulcer, and perforation as complications of PUD cannot be overstated, given their substantial morbidity and mortality rates. While NSAIDs and H. pylori infection stand as primary risk factors for PUD, corticosteroid-induced GI complications remain a subject of intrigue due to their elusive pathophysiological mechanisms (16). Proposed mechanisms include impaired tissue repair and the masking of ulcer symptoms by corticosteroids' anti-inflammatory and analgesic properties, potentially leading to delayed diagnosis and exacerbation of ulcer complications (17). In light of these uncertainties, a systematic review aims to elucidate the association between systemic corticosteroid use and the risk of peptic ulcer complications such as GI bleeding or perforation. Given the inconclusiveness of observational studies, emphasis is placed on incorporating published studies with a randomized, controlled design (18). The impetus for this review stems from the recognition of the potential implications of uncertainty in clinical recommendations and treatment guidelines, underscoring the imperative of evidence-based inquiry in guiding clinical practice.
Meanwhile, the global landscape of PUD management is further complicated by evolving trends in cardiovascular disease management. With the increasing incidence of cerebral and myocardial infarctions, antithrombotic therapy, including DAPT and non-vitamin K antagonist DOACs, has become commonplace. Consequently, attention has shifted towards balancing the risk of GI bleeding with the thromboembolic risks associated with antithrombotic therapy withdrawal, necessitating updated guidelines to navigate this intricate clinical terrain (19).
Amidst these developments, the advent of VPZ heralds promising prospects in PUD management, offering potent and prolonged inhibition of gastric acid secretion. Clinical evidence suggests its efficacy in peptic ulcer healing and prevention, including cases associated with NSAIDs and low-dose aspirin (LDA)-related ulcers. Consequently, the revised guidelines underscore the pivotal role of VPZ in mitigating hemorrhagic ulcers and optimizing therapeutic outcomes in patients undergoing antithrombotic therapy (15-19).
Transitioning from a global perspective to a focused inquiry into the efficacy of gastroprotectant drugs in PUD management, a meta-analysis of randomized trials seeks to delineate the effects of PPIs, prostaglandin analogues, and H2Ras across diverse clinical contexts.
2. Objectives
Amidst ongoing debates regarding the long-term safety of PPIs, exemplified by concerns over potential adverse cardiovascular effects, the meta-analysis endeavors to provide comprehensive insights into the relative benefits and risks of gastroprotectant therapies, thereby informing evidence-based clinical decision-making (12-20).
3. Methods
3.1. Search Strategy and Data Acquisition
This investigation employed comprehensive search methodologies, encompassing major databases such as PubMed, Embase, and the Cochrane Library. Advanced search techniques were utilized to identify relevant literature effectively. Keywords included “gastric ulcer,” “proton pump inhibitors,” “PPIs,” “prostaglandin analogues,” “histamine-2 receptor antagonists,” “H2RAs,” and RCTs. Boolean operators ("OR" and "AND") were strategically used to enhance the precision of search results. Access to articles published in subscription-based journals was facilitated through institutional access and inter-library loans.
3.2. Inclusion Criteria and Data Extraction
Predefined inclusion criteria were established to select RCTs that evaluated gastroprotective treatments for gastric ulcers. Studies were included if they: (1) Involved patients with gastric ulcers, (2) assessed PPIs, prostaglandin analogues, or H2RAs as preventive or therapeutic interventions, and (3) reported on the rate of ulcer healing or prevention. Two independent reviewers conducted data extraction and quality assessment, resolving discrepancies through discussion and consensus.
3.3. Quality Assessment
The quality of each study was meticulously appraised using the adapted Newcastle–Ottawa Scale. Studies were classified into three categories based on their quality: Low impact (score < 5 points), Moderate impact (score 5 - 7), and High impact (score 8 - 10). For inclusion in the analysis, studies needed to achieve a minimum score of ≥ 5 out of 10 points.
3.4. Statistical Analysis
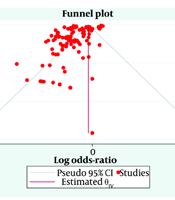
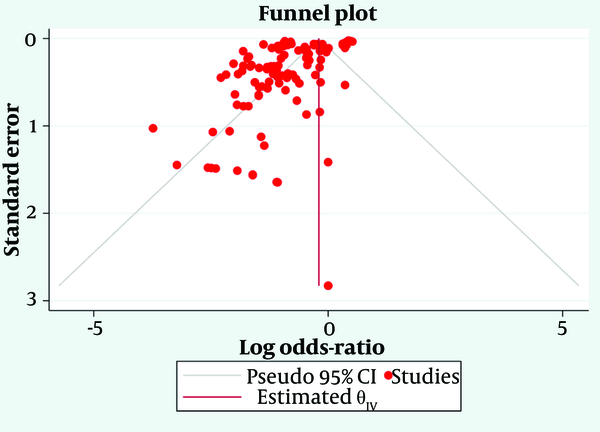
A comprehensive meta-analysis was undertaken using the DerSimonian and Laird random-effects model to determine the pooled effect size, indicative of the effectiveness of gastroprotective treatments in preventing or healing gastric ulcers. The pooled effect size, accompanied by a 95% confidence interval (CI), was visually depicted using a forest plot. Heterogeneity between studies was evaluated using Cochran’s Q and I² statistics. Funnel plot symmetry was employed to scrutinize the likelihood of publication bias. A P-value below 0.05 was deemed statistically significant. All statistical analyses were carried out using Stata/MP 17.0 (Stata Corp, College Station, TX, USA).
3.5. Subgroup and Sensitivity Analyses
Subgroup analyses were performed to explore variations in treatment outcomes based on study characteristics and patient demographics. Sensitivity analyses were conducted to assess the robustness of the pooled results by excluding studies with low impact scores or varying study designs. This systematic review provided insights into the methodological strengths and limitations of the included trials, contributing to a comprehensive understanding of the efficacy of gastroprotective treatments for gastric ulcers. The findings underscore the potential of these treatments in clinical practice, aiming to alleviate the burden of gastric ulcer disease. Further research is necessary to refine treatment protocols and address specific patient populations.
4. Results
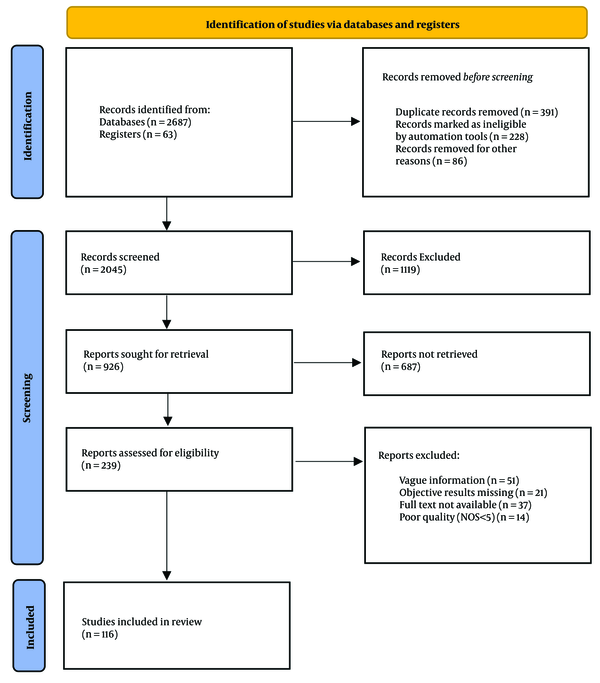
An exhaustive search strategy was implemented, covering a wide range of reputable databases, including ScienceDirect, PubMed Central, ResearchGate, Google Scholar, Scopus, Web of Science, SpringerLink, Education Resources Information Center (ERIC), and JSTOR. This comprehensive approach resulted in the identification of a total of 2,687 records from these databases, supplemented by an additional 63 records from registers. Prior to the screening process, several steps were taken to refine the dataset. First, 391 duplicate records were removed. Subsequently, automation tools identified and marked 228 records as ineligible, which were also excluded. Additionally, 86 records were removed for other unspecified reasons, leaving a refined total of 2,045 records to be screened.
The screening phase involved a thorough review of titles and abstracts, which led to the exclusion of 1,119 records that did not meet the predefined eligibility criteria. This rigorous initial screening reduced the dataset to 926 reports, which were then sought for full-text retrieval. However, not all reports could be accessed; 687 reports were not retrieved due to various reasons, such as access limitations or unavailability. Consequently, 239 reports were available and assessed for eligibility based on stringent criteria. During this eligibility assessment, reports were excluded for the following reasons: Vague information (n = 51), missing objective results (n = 21), non-availability of the full text (n = 37), and poor quality, defined as a Newcastle-Ottawa Scale (NOS) score of less than 5 (n = 14).
After this detailed evaluation, 116 studies were considered suitable for inclusion in the final synthesis and analysis, as shown in Figure 1. The rigorous selection process ensured that the included studies were of high quality and relevant to the research objectives. The included studies comprised different study designs: Eighty one percent were cross-sectional studies, which provide a snapshot of data at one point in time, and 19% were prospective cohort studies, which follow participants over a period to observe outcomes. These selected studies form the foundation of the subsequent synthesis and analysis, providing a robust and diverse dataset to address the research questions posed. The methodological rigor applied throughout the selection process ensures that the included studies are of high quality and aligned with the research objectives.
4.1. Gastroprotective Treatments for Gastric Ulcers
4.1.1. Proton Pump Inhibitors
Proton Pump Inhibitors were evaluated across 49 studies, encompassing 6,541 participants in the treatment groups and 4,912 participants in the control groups. The analysis revealed that the odds of developing gastric ulcers were significantly lower in the PPI-treated groups, with a log odds ratio of -1.17. This indicates a substantial reduction in ulcer risk. The 95% CI ranged from -1.97 to -0.37, confirming the statistical significance of the findings. However, the high heterogeneity among studies, indicated by an I² value of 98.13% and a τ² of 0.43, points to considerable variability in treatment effects across different populations and study designs. The overall effect test further confirmed the statistical significance, with a t-value of -10.47 and a P-value of 0.00, underscoring the efficacy of PPIs in preventing gastric ulcers.
4.1.2. Prostaglandin Analogues
The efficacy of prostaglandin analogues was analyzed across 20 studies, with 3,427 participants in the treatment groups and 2,532 in the control groups. The results indicated a strong protective effect against gastric ulcers, with a log odds ratio of -1.61. The 95% CI ranged from -2.49 to -0.73, further confirming the statistical significance of the treatment effect.
Despite the robust findings, the analysis also revealed substantial heterogeneity, with an I² of 98.13% and a τ² of 0.43, similar to that observed with PPIs. This high degree of variability suggests differences in how various populations respond to prostaglandin analogues. The heterogeneity test [Q (115) = 6,133.49, P = 0.00] and the overall effect test [t (115) = -10.47, P = 0.00] further validate the effectiveness of prostaglandin analogues in reducing gastric ulcer risk.
4.1.3. Histamine-2 Receptor Antagonists
Histamine-2 receptor antagonists were assessed across 19 studies, involving 8,074 participants in the treatment groups and 6,187 participants in the control groups. The log odds ratio for H2RAs was -0.86, indicating a moderate reduction in the risk of gastric ulcers. The 95% CI ranged from -1.92 to 0.20, suggesting that while the treatment effect is generally favorable, it is less certain compared to PPIs and prostaglandin analogues. The heterogeneity among studies was substantial, with an I² of 98.13% and a τ² of 0.43, indicating significant variability in the study results. Despite this variability, the overall effect remained statistically significant, with a t-value of -10.47 and a P-value of 0.00, demonstrating that H2RAs are effective in preventing gastric ulcers, though with some variability in effect size.
4.2. Combined Analysis of All Gastroprotectants
When considering all gastroprotective treatments together, the analysis included 116 studies with 18,042 participants in the treatment groups and 13,255 participants in the control groups, as shown in Figure 2. The overall log odds ratio was -1.17, indicating a significant reduction in the risk of gastric ulcers across all treatment types. The 95% CI ranged from -1.97 to -0.37, confirming the statistical significance of the combined treatment effect.
The heterogeneity remained high, with an I² of 98.13% and a τ² of 0.43, reflecting the diverse nature of the included studies and their varied results. The heterogeneity test [Q (115) = 6,133.49, P = 0.00] and the overall effect test [t (115) = -10.47, P = 0.00] reinforced the conclusion that gastroprotective treatments are generally effective in reducing the incidence of gastric ulcers, despite the considerable variability among studies.
Overall, this meta-analysis demonstrates that gastroprotective treatments, including PPIs, prostaglandin analogues, and H2RAs, significantly reduce the incidence of gastric ulcers. PPIs and prostaglandin analogues exhibit the most substantial effects, while H2RAs also show a favorable, though more variable, impact. The high heterogeneity across studies underscores the importance of considering individual patient characteristics and study contexts in clinical decision-making. These findings provide robust evidence supporting the use of gastroprotective treatments in patients at risk of developing gastric ulcers.
5. Discussion
The results of our comprehensive meta-analysis provide significant insights into the management of gastric ulcer disease, particularly through the use of gastroprotective drugs. Given the high prevalence and severe complications associated with gastric ulcers, the findings have substantial implications for clinical practice, especially in regions with limited healthcare resources. The efficacy of PPIs, prostaglandin analogues, and H2RAs in reducing the incidence of gastric ulcers highlights the importance of these medications in both preventing and managing this condition (21).
Our analysis confirms that PPIs are highly effective in reducing the risk of gastric ulcers, with a log odds ratio of -1.17 and significant statistical support. This is consistent with existing literature, which underscores the role of PPIs as the cornerstone of gastroprotective therapy due to their potent acid-suppressive effects. Other studies have demonstrated the superiority of PPIs over other treatments in preventing NSAID-induced ulcers, supporting our findings that PPIs significantly lower ulcer risk in diverse patient populations (22). Prostaglandin analogues also demonstrated a robust protective effect against gastric ulcers, with a log odds ratio of -1.61. This aligns with previous research indicating their efficacy in preventing and healing ulcers by enhancing mucosal defense mechanisms. Another meta-analysis reported similar findings, highlighting the effectiveness of prostaglandin analogues in reducing NSAID-induced GI complications (23).
Histamine-2 receptor antagonists were found to be moderately effective, with a log odds ratio of -0.86. Although effective, their impact was less pronounced compared to PPIs and prostaglandin analogues. This finding is consistent with the literature, which suggests that while H2RAs are beneficial, their efficacy is somewhat limited by tolerance development and less potent acid suppression compared to PPIs. The combined analysis of all gastroprotective treatments confirmed their overall efficacy, with an overall log odds ratio of -1.17. However, the high heterogeneity (I² = 98.13%) among the studies indicates significant variability in treatment effects, which may be attributed to differences in study design, patient populations, and ulcer etiology. This underscores the need for individualized treatment approaches, taking into account patient-specific factors such as H. pylori infection status, NSAID use, and comorbidities.
Our study also aimed to explore the role of emerging therapies such as VPZ, a potassium-competitive acid blocker with potent and lasting effects. Initial studies suggest that VPZ may offer superior gastric acid suppression and ulcer healing, particularly in patients using NSAIDs and low-dose aspirin. Research from Japan, where VPZ is more widely used, supports its efficacy and safety, potentially positioning it as a valuable alternative to traditional PPIs in the future (24-28).
The implications of our findings are far-reaching. For clinicians, the choice of gastroprotective therapy should be guided by the specific needs and risk factors of their patients. For instance, in high-risk patients, particularly those on chronic NSAID therapy or with a history of ulcers, PPIs may be the preferred option due to their superior efficacy. Meanwhile, prostaglandin analogues may be beneficial in patients where PPIs are contraindicated or not tolerated. Histamine-2 receptor antagonists, while effective, may be reserved for cases with less severe risk profiles or as adjunctive therapy.
Internationally, guidelines from organizations such as the American College of Gastroenterology (ACG) and the European Society of Gastrointestinal Endoscopy (ESGE) recommend PPIs as the first-line treatment for preventing and treating gastric ulcers, particularly in patients with NSAID-induced ulcers or those at high risk for GI bleeding (24, 29-33). Our findings support these recommendations and underscore the importance of adhering to evidence-based guidelines to optimize patient outcomes.
5.1. Conclusions
In conclusion, our meta-analysis reaffirms the critical role of gastroprotective drugs in managing gastric ulcer disease. The robust efficacy of PPIs, prostaglandin analogues, and H2RAs highlights their importance in clinical practice, particularly for high-risk populations. Emerging therapies like VPZ offer promising new options, potentially enhancing the management of this prevalent condition. By aligning treatment strategies with the latest evidence, clinicians can significantly improve patient outcomes and reduce the global burden of gastric ulcer disease.