1. Background
Human papillomavirus (HPV) is a small double-stranded DNA virus belonging to the Papillomaviridae family. It is associated with both benign and malignant clinical lesions and has a tropism for the squamous epithelium of the skin and mucosa. Human papillomavirus types are classified into five main genera—alpha, beta, gamma, mu, and nu. Based on epidemiological data, HPVs in the alpha HPV genus include low-risk cutaneous HPV (HPV2, 3, 10, 27, 28, 61, 62, 81), low-risk mucosal HPV (HPV6, 11, 13, 32, 42, 43, 74), probable high-risk (HR) HPV (HPV26, 53, 66, 67, 68, 70, 73, and 82), and HR mucosal HPV (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59) according to their oncogenic potential (1-3). High-risk HPVs are the causal agents of cervical cancer, anogenital cancer, and head and neck cancers. Beta, gamma, and mu HPVs primarily infect the skin epithelium (3).
Recent metagenomic analyses (4) have revealed that the highest prevalence of HPV is found in the skin (61%), followed by the vagina (41.5%), mouth (30%), and gut (17.3%). More than 95% of all viral sequences detected in skin samples belong predominantly to the beta and gamma HPV genera (5). Beta HPV types, such as HPV-5, HPV-8, and HPV-9, are common viruses that can lead to widespread infections, although they are typically asymptomatic. Persistent infections with beta HPV in keratinocytes have been shown to increase the risk of actinic keratosis. Beta HPV is present in the skin of 40% of immunocompetent individuals with squamous cell carcinoma (SCC) and in 80% of organ transplant recipients with SCCs. Additionally, beta HPV types 17, 20, and 38 appear to be significantly associated with SCCs (6).
Psoriasis is a chronic inflammatory dermatosis with a multifactorial etiology, affecting 2% of the global population. Psoriasis has a complex pathogenesis involving the skin microbiome as well as immunological and genetic factors (7). In the pathogenesis of psoriasis, which involves T cell-mediated inflammatory reactions, IL-17 produced by Th-17 is induced by HPV, leading to uncontrolled keratinocyte proliferation (8, 9). This creates a cell differentiation environment necessary for HPV to sustain its life cycle (10).
The treatment of psoriasis often requires systemic and local medications to improve patients' quality of life. Photochemotherapy, specifically with psoralen UV-A (PUVA), is a commonly used treatment method with immunosuppressive effects. The HPV is an opportunistic oncogenic virus frequently found in immunocompromised patients. Consequently, photochemotherapy may support immunosuppression and the proliferation of opportunistic HPVs (11).
2. Objectives
There is limited data regarding the etiological role of HPV genotypes in psoriasis cases. The present study aimed to investigate the presence of the alpha human papillomavirus genus in skin scrapings of psoriasis patients and to determine its genotype distributions.
3. Methods
3.1. Study Population and Sample Collection
The study used a cross-sectional design and included 53 psoriasis patients who presented to the Dermatology Outpatient Clinic of Mersin University Hospital between December 2020 and May 2021, along with 47 healthy individuals from Mersin province, Türkiye. The patients' clinical history and laboratory data were retrieved from hospital medical records, including age, sex, comorbidities, and treatments such as PUVA (0.5 - 2 J/cm2) and immunosuppressants (e.g., methotrexate, verxant, enbrel, etc.). Informed consent was obtained from all patients. The study was conducted in accordance with the declaration of helsinki, and the protocol was approved by the Mersin University Clinical Research Ethics Committee with decision number 768, dated 25/11/2020.
Individuals aged 18 and older who had been diagnosed with psoriasis and had no other skin diseases were included in the study. The study followed standard guidelines, involving the review of patient history, clinical examinations, and laboratory tests, with patients being randomly selected. The control group consisted of healthy individuals visiting the dermatology clinic who had no history of psoriasis or skin cancer and showed no clinical signs of viral warts. Additionally, it was confirmed that none of their first-or second-degree relatives had a history of psoriasis.
Skin scrapings were collected from both lesional areas (n = 53) and non-lesional areas (n = 39) of the body (hands, elbows, and back) in psoriasis patients, and from the elbows and arms of healthy individuals. Fourteen patients had psoriatic lesions spread across their entire bodies, preventing the collection of lesion-free area samples from these patients. Skin scrapings were collected from all participants using a sterile scalpel and placed in eppendorf tubes containing 0.9% NaCl. The samples were stored at -20°C until further examination.
3.2. Human Papillomavirus Detection and Genotyping
Nucleic acid isolation from skin scrapings was performed using the PureLink genomic DNA mini kit (Cat no. K1820-02, Invitrogen, USA) in accordance with the manufacturer's instructions. The presence of human genomic DNA in all samples was confirmed by amplifying a 136 bp fragment of the β-globin gene using GAPDH-F (5’-GGCAGCAGCAAGCATT CCT-3’) and GAPDH-R (5’-GCCCAACACCCCCAGTCA-3’) primers. Detection of HPV DNA was carried out using nested polymerase chain reaction (PCR) with consensus outer primers MY09 (5’-CGTCCMARRGGAWACTGATC-3’)/MY11 (5’-GCMCAGGGW CATAAYAATGG-3’) (M: A+C, R: A+G, W: A+T, Y: C+T) and inner primers GP5+ (5’-TTTGTTACTGTGGTAGATACTAC-3’)/GP6+ (5’-GAAAAATAACTGTAAATCATAT TC-3’). These primers target 450 bp and 142 bp fragments of the HPV L1 conserved region, respectively (12).
The PCR reaction mixture for both the first and second rounds included 10x PCR buffer, 2 μmol/μL MgCl2, 0.2 μmol/μL dNTP mix, 0.25 pmol/μL (sense and antisense) primers, 1.25 U Taq DNA polymerase (Thermo Fisher Scientific), and 5 μL of sample DNA in a total reaction volume of 50 μL. The PCR amplification conditions for each round were as follows: An initial denaturation at 94°C for 5 minutes, followed by 35 cycles of denaturation at 95°C for 45 seconds, annealing at 55°C (60°C for β-globin gene amplification) for 45 seconds, extension at 72°C for 1 minute, and a final extension step at 72°C for 7 minutes in a thermal cycler (Eppendorf Mastercycler, Hamburg, Germany).
Polymerase chain reaction products were separated by electrophoresis on a 1% agarose gel containing 0.5 µg/mL ethidium bromide and visualized using a UV transilluminator.
3.3. Identification of Human Papillomavirus Genotypes
The amplified second round of PCR products was subjected to cycle sequencing PCR using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA), which contains labeled dideoxynucleotides for inner sense and antisense chains. An automated DNA sequence analyzer, ABI PRISM 3130XL Genetic Analyzer (Applied Biosystems, Foster City, CA, USA), was used for the electrophoresis of the reaction products.
The obtained consensus sequences were uploaded to the National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLAST) software and compared with HPV reference sequence data in the GeneBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The identification of the HPV genotype was determined based on the closest and most significant alignment sequence matches.
3.4. Statistical Analysis
Categorical variables were expressed as numbers and percentages, while numerical variables were presented as mean ± standard deviation. The chi-squared test was used to assess relationships between categorical variables. Fisher’s exact test was applied when the expected frequency percentage of < 5 exceeded 25% during categorical data analysis. A P-value of < 0.05 was considered statistically significant.
4. Results
Among the psoriasis patients (n = 53), there were 27 males and 26 females, with mean ages of 50.9 ± 12.71 and 49.74 ± 12.46, respectively. The control group included 47 healthy individuals, comprising 25 males and 22 females, with mean ages of 36.37 ± 11.03 and 35.91 ± 10.94, respectively.
Among the psoriasis patients, seven (13.2%) had diabetes mellitus (DM), five (9.4%) had hypertension (HT), four (7.5%) had both DM and HT, and two (3.8%) had a history of breast cancer. No diagnoses of psoriatic arthritis or mental disorders were reported among these patients. In the control group, three (6.4%) of the 47 individuals had HT, and one (2.1%) had a history of diabetes mellitus.
A total of 22 psoriasis patients had received light therapy in previous years but were not actively undergoing PUVA treatment at the time of sampling (Table 1).
| Variables | Patients (n = 53) | Healthy Individuals (n = 47) | P-Value b |
|---|---|---|---|
| Age (y) | 49.8 ± 12.5 | 36.2 ± 11.0 | - |
| Gender | |||
| Female | 26 (49.0) | 22 (46.8) | |
| Male | 27 (51.0) | 25 (53.2) | |
| Comorbidity | - | ||
| HIV | - | - | |
| DM | 7 | 1 | |
| HT | 5 | 3 | |
| DM+HT | 4 | - | |
| Breast cancer | 2 | - | |
| Psoriatic arthritis | - | - | |
| Mental disorders | - | - | |
| No | 35 | 43 | |
| HPV vaccine | - | - | |
| HPV DNA | |||
| Positive | 18 (34.0) | 14 (29.8) | 0.823 |
| Lesion | 16 (30.2) | - | 0.018 |
| No-lesion | 2 (5.10) | - | |
| Negative | 35 (66.0) | 33 (70.2) | |
| PUVA (previously treated) | 0.697 | ||
| Yes | 22 (41.5) | - | |
| No | 31 (58.5) | - |
Abbreviations: HPV, human papillomavirus; DM, diabetes mellitus; HT, hypertension; PUVA, psoralen UV-A.
a Values are expressed as mean ± SD or No. (%).
b P-value < 0.05 was considered significant.
4.1. Human Papillomavirus DNA Detection by Polymerase Chain Reaction
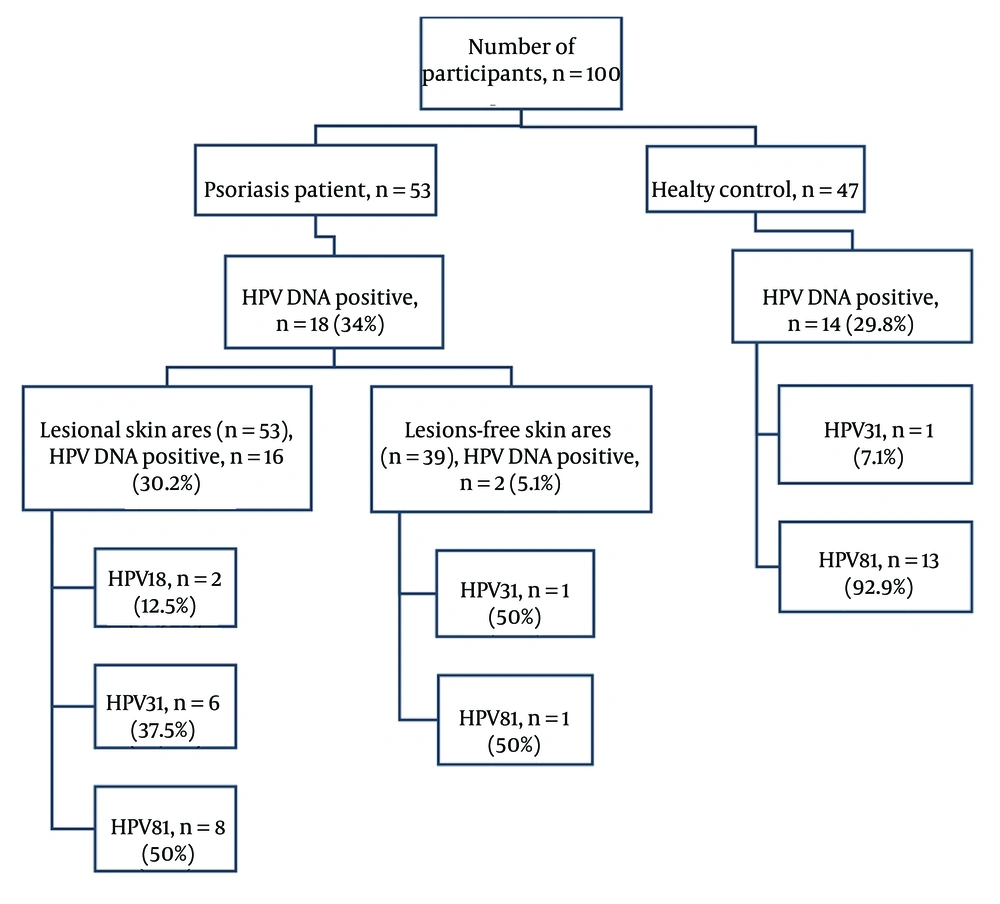
The HPV DNA was detected in 34% (18/53) of psoriasis patients in skin scrapings and 29.8% (14/47) of healthy individuals (P = 0.823). Among psoriasis patients, HPV DNA was identified in 30.2% (16/53) of skin scrapings from lesional areas and 5.1% (2/39) from non-lesional areas (P = 0.018). Psoriatic lesions had spread across the entire body in 14 patients, preventing the collection of samples from lesion-free areas. Skin scrapings from lesional and non-lesional areas of psoriasis patients were not concurrently positive. Demographic information for the patients and healthy individuals is provided in Table 1.
4.2. Human Papillomavirus Genotypes
The HPV18 was detected in 11.1% (2/18), HPV31 in 38.9% (7/18), and HPV81 in 50% (9/18) of skin scraping samples from psoriasis patients. The rates of HPV genotype distribution for lesional and non-lesional skin areas are shown in Figure 1. Among healthy individuals, HPV81 was found in 92.9% (13/14) and HPV31 in 7.1% (1/14) of the skin scrapings. The HPV31 was detected at a higher rate in psoriasis patients, whereas HPV81 was more prevalent in healthy individuals (P = 0.023) (Table 2).
| HPV Genotype | HPV DNA Positive | P-Value | |
|---|---|---|---|
| Patients (n = 18) | Healthy Individuals (n = 14) | ||
| HPV18 | 2 (11.1) | - | - |
| HPV31 | 7 (38.9) | 1 (7.10) | 0.023 |
| HPV81 | 9 (50.0) | 13 (92.9) | |
Abbreviation: HPV, human papillomavirus.
a Values are expressed as No. (%).
4.3. Psoralen UV-A-Human Papillomavirus Genotype Distribution
The HPV DNA was detected in 31.8% (7/22) of patients who had received PUVA treatment in previous years, with their genotype distribution being HPV18 (n = 2), HPV31 (n = 2), and HPV81 (n = 3). HPV DNA was detected in 35.5% (11/31) of patients who had never received PUVA treatment, with their genotype distribution being HPV31 (n = 5) and HPV81 (n = 6). No significant difference was found between the groups in terms of HPV DNA detection rates (P = 0.697) (Table 3).
| HPV Genotype | PUVA (Previously Treated) (n = 7) | PUVA Non-treated (n = 11) |
|---|---|---|
| HPV18 | 2 (28.6) | - |
| HPV31 | 2 (28.6) | 5 (45.5) |
| HPV81 | 3 (42.8) | 6 (54.5) |
| Total | 7 (100) | 11 (100) |
a Values are expressed as No. (%).
5. Discussion
The role of HPV in skin diseases has predominantly been attributed to beta HPVs, with limited data available on the relationship between alpha HPVs and skin diseases (13). This cross-sectional study investigated the presence and genotypes of alpha HPVs in psoriasis patients and healthy individuals. The rates and genotypes of alpha HPV in both groups, as well as the genotype distributions between them, were summarized. Although there are no similar studies in Türkiye, research from other countries has reported beta HPV DNA detection rates of 35.7% - 91.7% in the skin of psoriasis patients, emphasizing the potential involvement of HPV in the pathogenesis of psoriasis (14-18). In this study, the HPV DNA detection rate was 34% in the patient group. The lower detection rate in this study may be explained by its focus on alpha HPVs. Additionally, differences in viral prevalence between geographic regions may contribute to variations in detection rates (14).
While both alpha and other cutaneous HPVs are part of the healthy skin flora, the proportion of beta and gamma HPVs has been found to be higher than that of alpha HPVs (19). The prevalence of beta HPVs on intact skin of immunocompetent individuals has been reported to range from 18% - 70% (20-24), while that of alpha HPVs has been reported as 4.7% - 27.07% (13, 19, 25). Consistent with the literature, alpha HPV DNA was detected in 29.8% of healthy individuals in this study. No significant difference in the presence of HPV was observed between the patients and healthy individuals (P = 0.823). The virus is more prevalent in areas where inflammatory mechanisms are activated and keratinocytes overproliferate. Psoriasis patients commonly have lesions in areas with hyperproliferated keratinocytes. Prignano et al. demonstrated that HPV DNA was detected in 74.1% of psoriatic plaque scrapings from 54 patients and 33.3% of samples from lesion-free areas (14). Similarly, in this study, HPV DNA was detected in 30.2% of lesional skin and 5.1% of non-lesional skin samples from psoriasis patients (P = 0.018).
The genotypes detected in psoriasis patients and healthy individuals were the same, but the detection rates varied. Among psoriasis patients, HPV18 and HPV31, both of which have a well-established high oncogenic potential (25), were found in 11.1% and 38.9% of the patients, respectively, while the low-risk HPV81 was detected in 50%. In healthy individuals, HPV31 and HPV81 were detected in 7.1% and 92.9% of cases, respectively. Previous studies investigating the relationship between psoriasis and HPV reported beta and gamma HPV genotypes, including HPV5 (19.4% - 89.4%) (14 - 17,21), HPV36 (5.6% - 84.2%) (15-17, 21), HPV1 (42.1%) (15), HPV38 (14.8% - 24%), HPV25 (7% - 8.2%), HPV24 (1.6% - 7%), HPV14d (3.3%), HPV17 (3.3%) (17,18), HPV21 and HPV14 (21), HPV37 (3.3%), HPV51 (1.6%), HPV61 (1.6%), and HPV80 (1.6%) (18).
The inconsistencies between the results of this study and previous studies are likely due to differences in the primer sets used, the geographical origin of the study population, the sample type, the anatomical regions from which samples were collected, and the sample size.
In the present study, HPV DNA was detected in 31.8% of psoriasis patients who had received PUVA treatment in previous years and in 35.5% of those who had not received PUVA treatment (P = 0.697). High-risk HPV18 was detected in two patients who had received PUVA treatment, whereas no HPV18 genotype was detected in patients who had not received PUVA treatment. This finding suggests a possible association between immunosuppression caused by PUVA or biological agent use and HPV18 prevalence.
Consistent with the findings of Favre et al. (15), no significant correlation was observed between the detection rate of HPV DNA and the type of treatments received, including prior PUVA treatment, methotrexate, verxant, enbrel, or topical therapies. However, some studies in the literature suggest a potential link between immunosuppression caused by PUVA or the use of biological agents and increased HPV prevalence (11). These studies propose that photochemotherapy might contribute to immunosuppression, potentially facilitating the proliferation of opportunistic HPV strains. It is worth noting that in our study, patients who had previously received PUVA treatment were not undergoing active treatment at the time of sample collection, a factor that may have influenced the findings.
This study has several limitations. First, its cross-sectional design prevented the assessment of long-term persistence of HPV in the patients. Consequently, it was not possible to establish a relationship between the identified genotypes and disease severity. Another limitation was the focus on a limited number of HPV genotypes. While the literature includes limited references regarding the association between beta and gamma HPV types with cutaneous lesions and psoriasis, there is a lack of evidence concerning alpha HPV and its relationship with psoriasis. To address this gap, our study exclusively evaluated the presence of alpha HPV, excluding beta and gamma HPV types.
5.1. Conclusions
The present study provides valuable epidemiological data on the prevalence of alpha HPVs on the skin of psoriasis patients and healthy individuals. Psoriasis patients undergoing lifelong immunosuppressive treatment may face a potential risk from persistent HPV infections, particularly from HR oncogenic types (HPV18 and HPV31) identified in this study. Conversely, the high prevalence of low-risk HPV81 in healthy individuals suggests that alpha HPVs may be part of the normal skin flora without causing cutaneous lesions in immunocompetent individuals.
As there are no similar studies in Türkiye, these preliminary epidemiological findings are significant for understanding the potential impact of alpha HPVs on psoriasis patients in the region. Furthermore, the genotypes identified in this study may serve as a foundation for future research exploring their effects on keratinocyte differentiation and their role in the pathogenesis of skin conditions.