1. Introduction
Candida auris is an emerging fungal pathogen characterized by high virulence and substantial resistance to medications. Common hospital disinfectants are often ineffective against it, and it can persist on human skin and surfaces for months (1), leading to hospital-based transmission and infections, posing a significant threat to public health. Candida auris was first identified as a new Candida species in Japan in 2009, isolated from a patient’s ear canal. Since then, it has spread rapidly worldwide. In the United States, the first reported case occurred in 2016, and the number of C. auris cases has since shown dramatic increases: Fourty-four percent in 2019, 59% in 2020, and 95% in 2021 (2). Alarmingly, cases resistant to echinocandins—the first-line antifungal treatment—tripled in 2021 compared to the preceding two years (2).
As of 2023, the U.S. Centers for disease control and prevention (CDC) described C. auris as spreading at an "amazing speed," with cases reported in over half of U.S. states. In China, C. auris was first identified in 2018, with fewer than 100 cases reported initially, mainly in Shenyang, Xiamen, Beijing, and Guangdong (3). However, 2023 witnessed a significant surge in cases, emphasizing the urgent need for vigilance and control measures. Despite this increase, the incidence of C. auris infections in China remains lower than those reported in the U.S., South Africa, and India (4). In patients of any age, the risk of C. auris infection is heightened by prolonged ICU stays, underlying diseases, exposure to multiple antibiotics, and extensive medical interventions (5). For patients receiving antifungal treatment, the crude in-hospital mortality rate for C. auris candidemia is estimated to range between 30% and 70% (6).
With its rising incidence, C. auris has become a major pathogen in bloodstream infections (BSIs), surpassing C. albicans in some healthcare facilities (7). Additionally, C. auris presents challenges in identification and treatment, particularly in resource-limited settings, where diagnostic tools for C. auris may be unavailable and access to antifungals other than fluconazole is restricted. Given the clinical importance and global dissemination of this species, we report the first documented case of C. auris in Zhejiang Province and provide a brief phylogenetic analysis of the isolated strain.
2. Case Presentation
A 60-year-old male patient with a history of diabetes and hypertension was admitted to the ICU of Jinhua Hospital of Zhejiang University on November 27, 2023, due to severe pneumonia. Eleven months earlier, the patient had been diagnosed with elevated creatinine levels and renal insufficiency at Nanjing Tongren Hospital, where he was hospitalized for treatment. Two months prior to his current admission, the patient reported chest tightness during activity and at night, along with bilateral lower limb edema and fatigue. He returned to Nanjing Tongren Hospital, where further examinations revealed bilateral lung infections. One month before admission to Jinhua Hospital, the patient developed unconsciousness and shortness of breath, necessitating ICU care, where he received mechanical ventilation, dialysis, closed thoracic drainage, and anti-infective treatments. These interventions led to slight improvement in his consciousness.
For continued treatment, the patient was transferred to the ICU of Nanjing Zhongda Hospital, but his condition remained critical. Seeking further intensive care, the patient’s family arranged for him to be transferred to Jinhua Hospital to spend his final days in his hometown, Jinhua. Given the patient’s prolonged hospitalization and the potential for carrying multidrug-resistant organisms (MDRO), he was admitted to a single room in the ICU. Upon admission, the patient exhibited no pupillary light reflex, was intubated, and placed on mechanical ventilation. A hemodialysis catheter was inserted in the left femoral vein.
CT scans revealed multiple lung infections, and blood tests showed elevated white blood cell (WBC) counts at 19.59 × 10⁹ L, with a neutrophil percentage of 87.1%. C-reactive protein (CRP) and procalcitonin (PCT) levels were significantly increased, at > 200.00 mg/L and 1.18 ng/mL, respectively. Given the severity of the patient’s condition, comprehensive diagnostic tests were conducted, including two sets of blood cultures, sputum culture, Galactomannan (GM test), 1,3-β-D-glucan (G test), and respiratory viral nucleic acid tests. In the absence of a confirmed pathogen, empirical treatment was initiated. The regimen included imipenem combined with voriconazole for antimicrobial and antifungal coverage and oseltamivir for antiviral therapy.
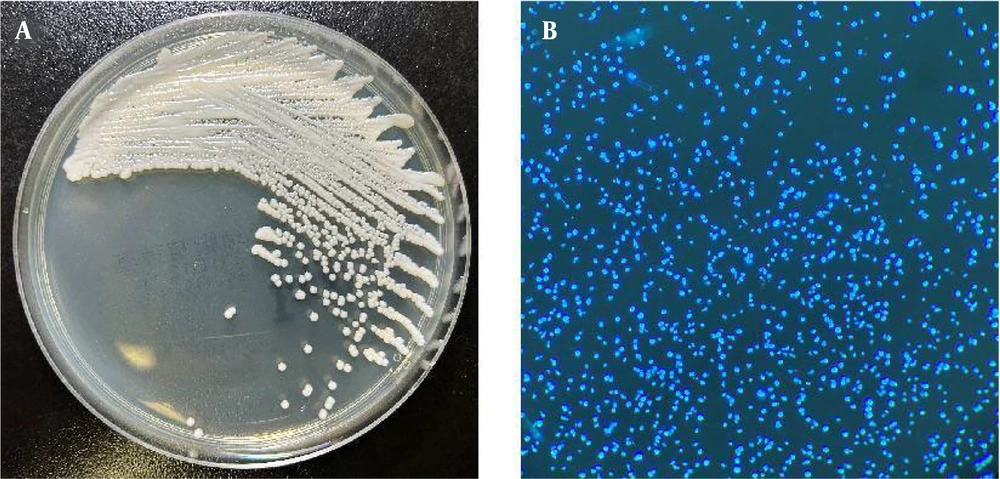
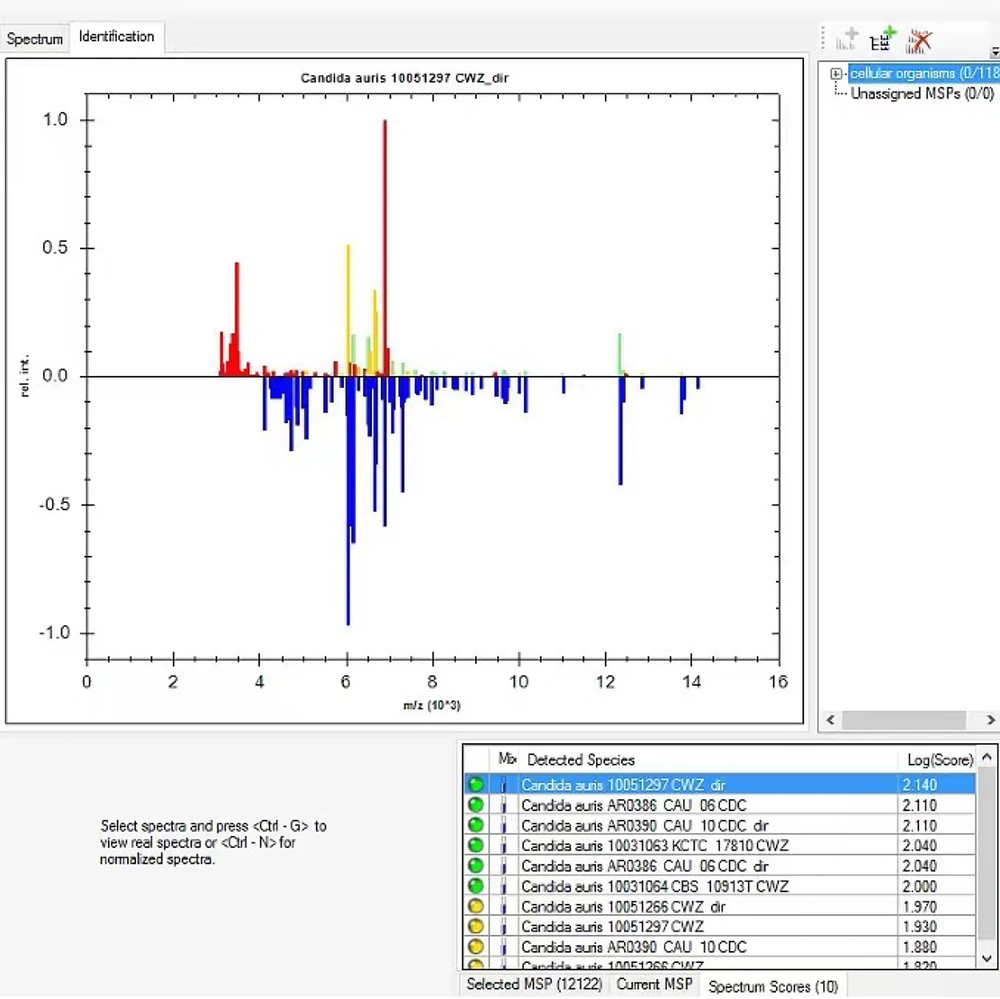
The following day, gram-negative bacilli were isolated from the left anaerobic blood culture bottle and identified as Escherichia coli, with susceptibility testing indicating sensitivity to imipenem. The respiratory viral nucleic acid test returned negative, though the possibility of a false-negative result was considered. The initial anti-infective and antiviral regimen was maintained. On the third day, E. coli with the same susceptibility profile as that found in the blood culture, multidrug-resistant Acinetobacter baumannii (MDRA), and a yeast-like fungus (Figure 1) were detected in the sputum. The yeast-like fungus was identified as C. auris using the MALDI-TOF MS system (Bruker Daltonik, Germany) (Figure 2). Antifungal susceptibility testing, performed using the yeast-like fungi Xinke system (Xinke Biological, Shandong, China), demonstrated high-level resistance to fluconazole in C. auris (Table 1).
Comparison results of Candida auris by the Bruker database (MALDI Biotyper). Comparison results of C. auris by the Bruker database (MALDI Biotyper IVD database version 13). The log score being 2.140 (according to the manufacturer's instruction manual, a score > 2.0 indicates safe genus identification and possible species identification).
| Antifungals | Range (μg/mL) | MIC (μg/mL) | CDC Tentative Resistance Breakpoints (μg/mL) |
|---|---|---|---|
| Fluconazole | 0.5 - 64 | > 64 | ≥ 32 |
| Itraconazole | 0.03 - 8 | 0.25 | Consider using fluconazole susceptibility as a surrogate for second generation triazole susceptibility assessment |
| Voriconazole | 0.03 - 4 | 0.5 | |
| 5-fluorocytosine | 0.125 - 32 | 0.125 | Not determined |
| Amphotericin B | 0.03 - 8 | 1 | ≥ 2 |
| Micafungin | 0.03 - 4 | 0.125 | ≥ 4 |
| Caspofungin | 0.03 - 4 | 0.125 | ≥ 2 |
Antifungal Susceptibility Profile of Candida auris Isolated from This Case, and Comparison with Control and Prevention Tentative Resistance Breakpoints
In vitro antifungal susceptibility testing was conducted using broth microdilution, following the Clinical and Laboratory Standards Institute (CLSI) M27-A3 standard (8). Despite the detection of C. auris, the GM and G test results in serum samples were negative, and clinicians decided not to modify the antifungal treatment for C. auris. The respiratory viral nucleic acid test was rechecked and remained negative, prompting the discontinuation of oseltamivir. Blood tests revealed a further increase in inflammatory markers, with a WBC count of 21.29 × 10⁹ L, CRP at 189.00 mg/L, and PCT at 15.83 ng/mL, indicating worsening inflammation. To intensify anti-infective therapy, tigecycline 100 mg ivgtt q12h was added to the treatment regimen.
On the fourth day, C. auris was again detected in a sputum culture. The patient experienced sudden cardiac arrest, and immediate resuscitation efforts were initiated, including chest compressions and intravenous administration of 1 mg of epinephrine every 4 minutes. The patient’s sinus rhythm was restored, but his overall condition—including lung infection, respiratory failure, septic shock, cardiac arrest, and hypoxic-ischemic encephalopathy—continued to decline. After discussions with the patient’s family regarding the poor prognosis, they decided to discontinue further treatment. The patient was subsequently discharged.
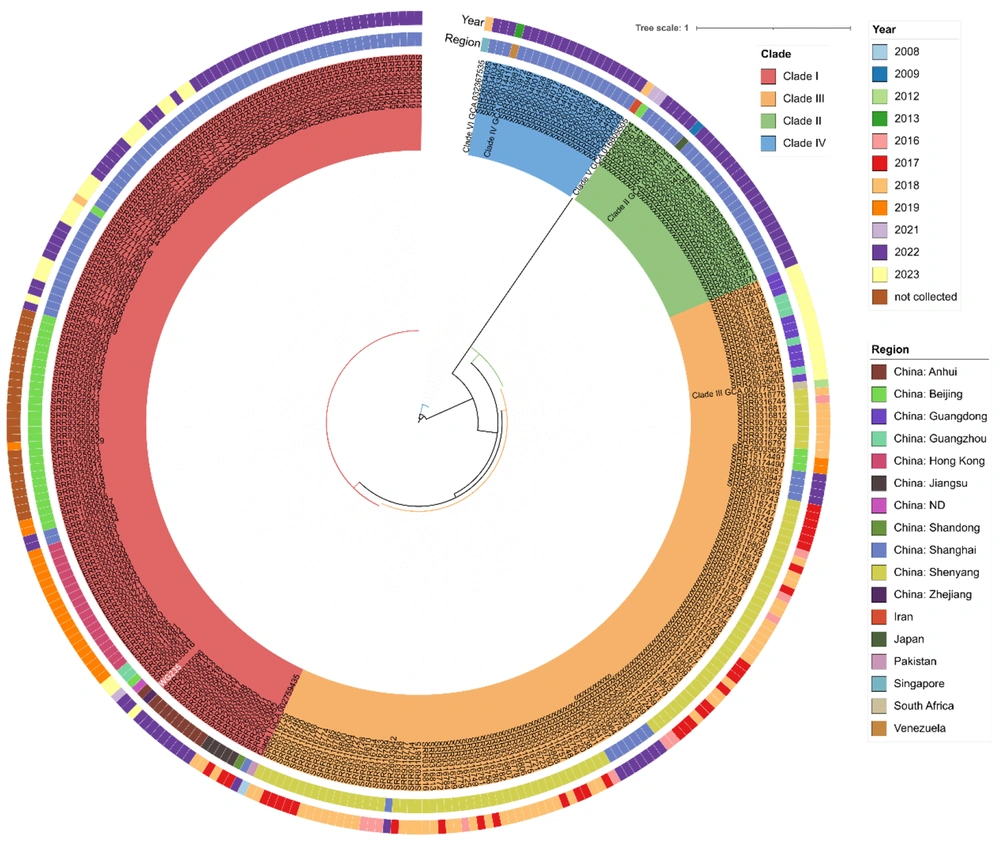
Whole genome sequencing (WGS) and phylogenetic analysis were conducted on the isolate. Genomic DNA was extracted using the Yeast Genomic DNA Rapid Extraction Kit (Sangon Biotech, China). Total DNA was processed with Nextera XT library preparation and paired-end sequenced on an Illumina NextSeq 2000 system (Illumina Inc., USA) (2 × 150 bp) following the manufacturer’s instructions. The isolate was confirmed as C. auris through WGS, and phylogenetic analysis revealed that the strain belonged to the South Asian-related clade I (Figure 3). Surveillance cultures and environmental swabs from other ICU patients were negative for C. auris, and no additional cases of C. auris infection or colonization were identified within the hospital.
Phylogenetic analysis of Candida auris (WG235) isolated from this case. Phylogenetic tree showing the genetic relationships among isolated strains representing different clades. The innermost large circle shows isolated strains of clade I, II, III, and IV, indicated in red, Green, pink, orange, and blue, respectively. The middle large circle shows the regional distributions of each strain, each colored bar corresponding to one region. The outermost large circle shows the year distributions of strains isolated from each region, each colored bar corresponding to a year.
3. Discussion
In the past decade, cases of C. auris infections have risen exponentially, spreading to over 50 countries across six continents (9), with particularly high prevalence in the U.S. and India, posing a significant threat to global health. Candida auris is geographically divided into four clades: Clade I (South Asia), clade II (East Asia), clade III (South Africa), and clade IV (South America) (10). Currently, three clades—Clade I, II, and III—are circulating in China (6). Clade I, III, and IV strains are commonly associated with multidrug resistance and nosocomial outbreaks, whereas clade II strains are typically susceptible to all antifungal drugs and have not been linked to outbreaks (11).
The isolate in this study belonged to clade I and was resistant only to fluconazole, a finding consistent with other studies in China (4). Patients infected or colonized with C. auris almost always present with multiple underlying diseases or comorbidities, including diabetes, sepsis or BSIs, pulmonary diseases, chronic or acute kidney failure, malignancies, cardiovascular diseases, and liver disease. In this case, the C. auris isolate was detected in a patient with severe pneumonia who had multiple underlying diseases: Diabetes, hypertension, and renal insufficiency. This profile aligns with other reported cases in the literature (12), where critically ill patients with multiple comorbidities were identified as being at high risk for C. auris colonization or infection.
The patient had been treated in several hospitals before being transferred to the ICU of our hospital, where sputum culture and other diagnostic tests were performed on the first day of admission, alongside empirical treatment with imipenem, voriconazole, and oseltamivir. On the third day, white colonies growing on a CHROMagar plate were identified by MALDI-TOF MS as C. auris, highlighting the importance of this technology for accurate and rapid identification.
There are no specific established antifungal susceptibility breakpoints for C. auris; however, the CDC has proposed tentative resistance breakpoints (13). Echinocandins are generally recommended as the initial antifungal therapy for C. auris, but in this case, the patient was treated with voriconazole based on susceptibility testing. Despite the low MIC value for voriconazole, the patient’s condition continued to deteriorate, with inflammatory markers rising. This lack of improvement could be attributed to the patient’s overall health status, compromised immunity, severity of infection, or the choice of antifungal therapy.
Clinicians decided not to modify the antifungal regimen, and while C. auris was again detected in a sputum culture on the fourth day, the patient’s worsening condition and the family’s decision to discontinue treatment precluded further adjustments. In this case, the precise role of C. auris in the clinical aggravation and multiorgan failure remains unclear. However, the association of C. auris with higher mortality rates underscores the pathogen’s potential contribution to the unfavorable outcome.
Candida auris is challenging to identify and is associated with strong infectivity, multidrug resistance, immune evasion, and high fatality rates in BSIs. Accurate and early identification of this pathogen is critical for improving patient outcomes and preventing transmission. The microscopic morphology of C. auris is not distinctive, and it forms polychromatic colonies (white, pink, or purple) on CHROMagar plates, complicating its identification through conventional laboratory methods. Candida auris isolates are often misidentified as C. haemulonii or Rhodotorula glutinis by automated yeast identification systems such as Vitek2 (14). These identification challenges likely result in significant underestimation of C. auris infections in healthcare facilities across China.
Molecular testing methods, including PCR and MALDI-TOF MS with updated databases, are the most effective and widely used diagnostic tools. Systems like the Bruker Biotyper™ and Vitek MS can detect C. auris with 100% sensitivity and specificity within minutes (12). Although WGS is not a routine diagnostic method for C. auris, it serves as a reference for strain-level identification and is vital for epidemiological analyses during outbreaks. In this case, MALDI-TOF MS accurately identified C. auris, demonstrating its utility as a fast, precise, convenient, and cost-effective diagnostic tool. Candida auris can colonize and spread in hospital environments, surviving on wet or dry surfaces for at least seven days and persistently on plastic surfaces for at least two weeks (15). Its ability to survive in various conditions and spread easily necessitates regular monitoring and disinfection of potentially contaminated healthcare facilities.
In this case, the patient’s prolonged hospitalization in multiple hospitals and potential carriage of MDRO led to the implementation of protective measures upon admission. The patient was housed in an isolation room, and staff utilized personal protective equipment. When C. auris was isolated, the patient was already under surveillance by the local Infection Prevention team. Safety measures were subsequently reinforced, including reducing equipment sharing, strictly adhering to hand hygiene practices, and regularly disinfecting the entire ICU. These measures successfully prevented further transmission, with no additional C. auris isolates reported. Given that the patient had been previously treated in the ICU of hospitals in Nanjing and that C. auris was detected only after transfer to the ICU of Jinhua Hospital, Zhejiang University, it is plausible that the infection or colonization occurred in one of the Nanjing hospitals.
In summary, this case highlights the critical importance of appropriate prevention and control measures, timely identification, and individualized treatment strategies for C. auris, particularly in critically ill patients. The timely and accurate detection of C. auris by MALDI-TOF MS is of significant value in preventing its spread. These findings emphasize the necessity for routine monitoring and preparedness in healthcare facilities to manage C. auris outbreaks and reinforce the role of MALDI-TOF MS as a frontline diagnostic tool in settings where C. auris is suspected or identified.
3.1. Conclusions
To date, this is the first reported case of clade I C. auris in Zhejiang. As an advanced microbial detection tool, MALDI-TOF MS enables rapid and accurate identification of C. auris. Based on the patient’s hospitalization history, it is plausible that this C. auris isolate was acquired from the hospital in Nanjing, highlighting the risk of inter-hospital transmission due to the convenience of medical transfers. Recommendations: (1) Critically ill patients with prolonged ICU stays and multiple underlying diseases are at increased risk of C. auris infection and should be routinely screened for C. auris with appropriate preventive and control measures implemented; (2) upon detection of C. auris, immediate contact precautions, routine monitoring, and thorough disinfection of potentially contaminated healthcare facilities are essential; (3) with the rising epidemic of C. auris in China, clinicians should enhance their diagnostic and therapeutic capabilities for managing C. auris infections.