1. Background
Hepatitis B virus (HBV) infection is a widespread and serious public health issue globally, posing a significant threat to global health (1). The prevalence of HBV infection varies between 2 - 8% worldwide, depending on endemic characteristics. Accordingly, countries with a prevalence of 8% and above are categorized as having high endemicity, those with a prevalence between 2 - 7% are classified as having moderate endemicity, and those with a prevalence below 2% are considered as having low endemicity. With a prevalence of 4.7%, our country is considered as having moderate endemicity (2). The natural course of HBV infection is determined by the balance between viral replication and the host's immune response. Therefore, the clinical diagnosis of HBV infection is achieved through serological detection of HBV antigens and antibodies produced by the host. During HBV infection, four structural antigen-antibody systems are observed: Hepatitis B surface antigen (HBsAg) and antibody (anti-HBs); pre-S antigens associated with HBsAg and their antibodies; hepatitis B core antigen (HBcAg) and anti-HBc; and hepatitis B e antigen (HBeAg) and anti-HBe. Examination of these antigen and antibody systems enables the diagnosis of hepatitis B virus infection and monitoring of the disease course (3).
Following exposure to the hepatitis B virus, the virus persists intracellularly as cccDNA. In cases of low or undetectable HBV viremia, anti-HBc can methylate intracellular cccDNA, reducing DNA and mRNA expression. Additionally, downregulation of acetylation can lead to suppression of cccDNA, resulting in atypical profiles such as "isolated hepatitis B core antibody (isolated anti-HBc)" patterns (4). Serological test results that deviate from the norm in hepatitis B virus infections require advanced examination and evaluation due to challenges in interpretation during diagnosis and follow-up. Among the unusual serological profiles, isolated anti-HBc positivity is the most commonly observed (5).
A serological case where HBsAg is negative, anti-HBs is negative, and anti-HBc is positive is defined as isolated anti-HBc positivity, and the frequency of isolated anti-HBc varies between 0.1 - 20% in various populations (6, 7). Since HBV screening in most countries relies primarily on testing for HBsAg and/or anti-HBs, instances of isolated anti-HBc positivity are often disregarded. However, in terms of public health, the infectivity of the disease in individuals with isolated anti-HBc positivity is an important problem. This is because, while HBV infection appears to be nonexistent in this profile due to HBsAg negativity, in patients included in this profile, there are special cases showing that the disease is infective, such as the window period of acute infection and occult HBV (8).
Firstly, isolated anti-HBc positivity may arise years after a resolved hepatitis B infection, when anti-HBs antibodies decrease to undetectable levels (4, 9-11). Secondly, this pattern can also occur during the recovery phase of the infection, when HBsAg has disappeared but anti-HBs antibodies are not yet detectable. However, in these cases, anti-HBc IgM positivity accompanies the pattern (2). This scenario indicates the window period of the infection. Thirdly, isolated anti-HBc patterns may be observed in some cases of occult HBV infections. To confirm the diagnosis, HBV DNA should be examined in liver biopsy samples and/or serum.
Although clinical guidelines have existed for some time, there is still much debate about the management of isolated anti-HBc-positive patients at risk of hepatitis B reactivation. At this time, the most reliable test used to detect HBV reactivation is a one-log increase in serum HBV DNA concentrations. When HBV reactivation is diagnosed, it is recommended that antiviral treatment be started immediately. Prophylactic treatment, on the other hand, is usually recommended for patients with a high risk of reinfection/reactivation. For patients in the moderate-risk and low-risk groups, in addition to hepatitis B vaccination, regular ALT, AST, HBsAg, and HBV DNA monitoring and the initiation of treatment in the case of reactivation are recommended (7, 12).
2. Objectives
This study determined the prevalence of isolated anti-HBc positivity in samples sent to the medical microbiology laboratory for hepatitis B indicators and identified the rates of anti-HBc IgM and HBV DNA positivity within this pattern. Additionally, it explored the association of this atypical pattern with different clinical scenarios, aiming to contribute to our country's data on the subject.
3. Methods
From January 2015 to July 2023, data were retrospectively collected from serum samples sent to the Trakya University Medical Microbiology Laboratory for HBsAg, anti-HBs, anti-HBc, anti-HBc IgM, and HBV DNA testing, along with demographic information of the patients. Hospital information systems and patient files were reviewed for this purpose. Patients with any missing data for HBsAg, anti-HBs, or anti-HBc parameters were excluded from the study. Results from the tests ordered by different physicians on different dates were not combined. Each patient was included once in the study.
Serological status was defined as isolated anti-HBc positivity for patients negative for HBsAg and anti-HBs but positive for anti-HBc. Patients in whom HBsAg, anti-HBs, anti-HBc, and anti-HBc IgM were tested simultaneously were examined, and the rate of anti-HBc IgM positivity in patients with isolated anti-HBc positivity was determined. Additionally, patients in whom HBsAg, anti-HBs, anti-HBc, and HBV DNA were tested simultaneously were examined, and the rate of HBV DNA positivity in patients with isolated anti-HBc positivity was determined. If multi-stage tests were ordered for anti-HBc IgM and HBV DNA, the case was excluded.
Hepatitis B surface antigen, anti-HBs, anti-HBc, and anti-HBc IgM tests were conducted using Cobas e411 and e611 analyzers (Roche Diagnostics GmbH, Germany) from 2015 to 2023, and the Architect i2000 analyzer (Abbott, USA) in 2023. HBV DNA testing was performed using the Montania 4896 real-time PCR device (Anatolia Diagnosis and Biotechnology, Turkey) from 2015 to 2023, and the COBAS TaqMan 48 system (Roche, USA) in 2023. The data were analyzed using IBM statistical package for social sciences (SPSS) statistics 21.0 program. Descriptive statistics such as number, percentage, mean, and standard deviation were utilized in evaluating the results of the study.
The normal distribution of variables was assessed using the Kolmogorov-Smirnov test. The data were analyzed for differences between groups using the chi-square test. Results were considered statistically significant at P < 0.05. In multivariate analysis, logistic regression analysis was employed using potential factors identified in previous analyses. The goodness of fit of the model was assessed using the Hosmer-Lemeshow test. In the Hosmer-Lemeshow test, cases where the P-value is greater than 0.05 indicate models with high predictive value. R2 is the correlation coefficient, and the closer it is to 1, the better the model's fit.
4. Results
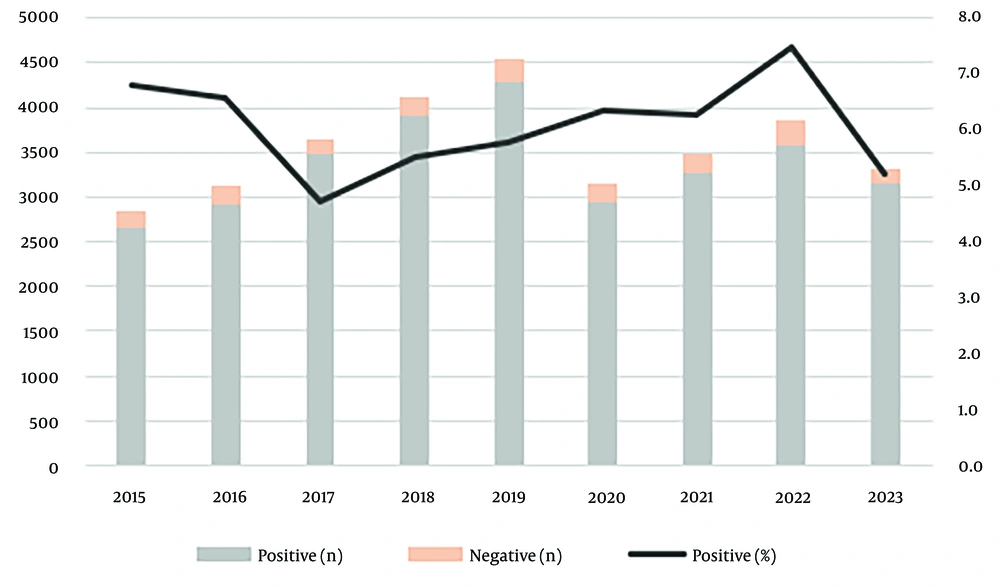
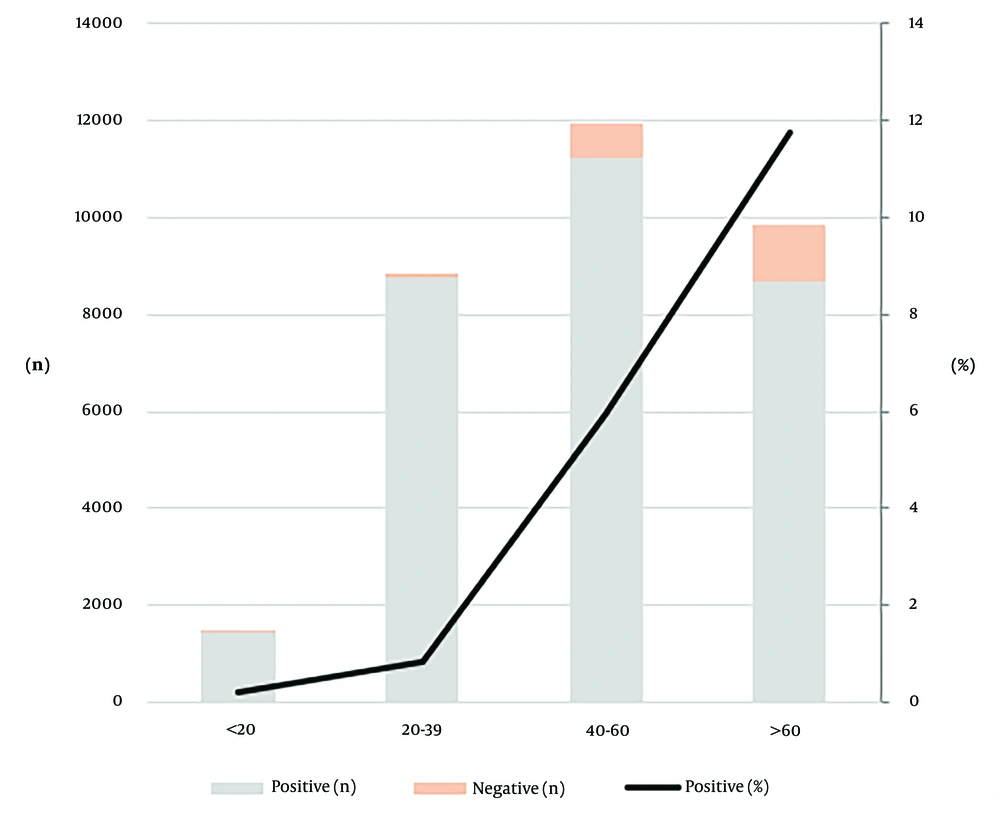
Between January 2015 and July 2023, a total of 36,216 patients were tested for anti-HBc, of which 14,980 (41.4%) were positive and 415 (1.1%) were borderline. Among 32,097 patients tested simultaneously for HBsAg, anti-HBs, and anti-HBc, isolated anti-HBc positivity was observed in 1,941 (6.0%) patients (Table 1 and Figure 1). The mean age of the patients was 49.02 ± 18.49 years. The mean age of male patients with isolated anti-HBc positivity was 63.2, while for female patients, it was 62.5. Isolated anti-HBc positivity was observed in < 20 years old patients at a rate of 0.2%, in patients aged 20 - 40 years at 0.8%, in patients aged 40 - 60 years at 5.7%, and in patients aged > 60 years at 11.6% (Figure 2).
| Variables | No. (%) |
|---|---|
| Year | |
| 2015 | 2845 (8.9) |
| 2016 | 3117 (9.7) |
| 2017 | 3650 (11.4) |
| 2018 | 4124 (12.8) |
| 2019 | 4543 (14.2) |
| 2020 | 3145 (9.8) |
| 2021 | 3484 (10.9) |
| 2022 | 3870 (12.1) |
| 2023 | 3319 (10.3) |
| Unit | |
| Outpatient | 24666 (76.8) |
| Inpatient | 7431 (23.2) |
| Department | |
| Internal medical | 30260 (94.3) |
| Surgical medical | 1837 (5.7) |
| Sex | |
| Female | 16524 (51.5) |
| Male | 15573 (48.5) |
| Isolated anti-HBc | |
| Positive | 1941 (6.0) |
| Negative | 30156 (94.0) |
Abbreviation: HBc, hepatitis B core.
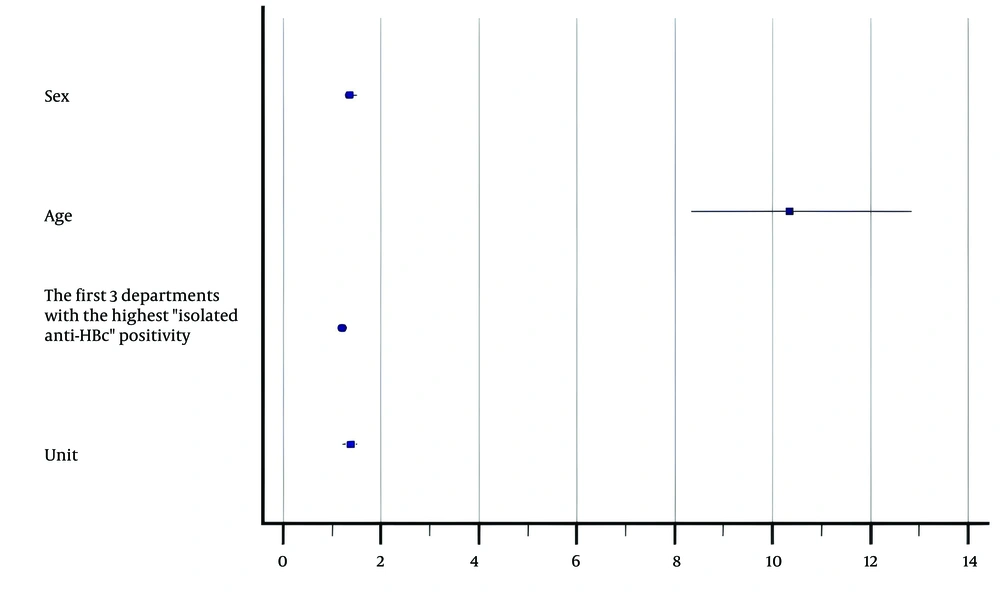
The rate of isolated anti-HBc positivity was 7.1% in males and 5.0% in females. Isolated anti-HBc positivity was most frequently detected in samples from the gastroenterology (21.3%), oncology (16.6%), and internal medicine (12.3%) departments, followed by hematology (10.3%), rheumatology (7.3%), infectious diseases (5.9%), and nephrology (5.2%) departments. Some characteristics of patients with isolated anti-HBc positivity are shown in Table 2. Isolated anti-HBc positivity was found to be associated with male gender (1.36 times), being a patient of the top three departments where positivity was most frequently detected (1.20 times), being an outpatient (1.37 times), and being over 40 years old (10.34 times) (Figure 3).
| Variables | Positive | Negative | P-Value |
|---|---|---|---|
| Unit | 0.000 a | ||
| Outpatient | 1287 (66.3) | 23379 (77.5) | |
| Inpatient | 654 (33.7) | 6777 (22.5) | |
| Department | 0.314 | ||
| Internal medical | 1820 (93.8) | 28440 (94.3) | |
| Surgical medical | 121 (6.2) | 1716 (5.7) | |
| Sex | 0.000 a | ||
| Female | 829 (42.7) | 15695 (52.0) | |
| Male | 1112 (57.3) | 14461 (48.0) | |
| Age | 0.000 a | ||
| ≤ 40 | 90 (4.6) | 10661 (35.4) | |
| > 40 | 1851 (95.4) | 19495 (64.6) | |
| The first 3 departments with the highest isolated anti-HBc positivity and others | 0.000 a | ||
| Gastroenterology, oncology, internal medicine | 975 (50.4) | 12490 (41.7) | |
| Others | 960 (49.6) | 17476 (58.3) |
Abbreviation: HBc, hepatitis B core.
a P < 0.05.
Among the 873 patients with isolated anti-HBc positivity in whom anti-HBc IgM was tested, 5 (0.6%) were positive. Four of these patients were male, one was female, and their mean age was 49.6 years (min = 25, max = 65). In the patients with isolated anti-HBc positivity, 22 (8.4%) of the 262 patients who were tested for HBV DNA were positive. Among the HBV DNA-positive patients, 72.7% were male, and 27.3% were female, with a mean age of 59.3 years (min = 38, max = 77). Of these patients, 9 (40.9%) were referred by gastroenterology, 4 (18.2%) by internal medicine, 4 (18.2%) by hematology, 3 (13.6%) by infectious diseases, and 2 (9.1%) by other departments. The results of all patients with HBV DNA positivity in our study were < 3000 IU/mL, with 86.4% having values < 100 IU/mL.
5. Discussion
Hepatitis B virus infection poses a serious global health issue. Approximately 257 million individuals worldwide are infected with HBV, and the number of people who have been exposed to HBV at some point reaches 2 billion, making hepatitis B one of the most prevalent infections globally (8, 13). Isolated anti-HBc serological pattern is defined as the condition where anti-HBc is positive while HBsAg and anti-HBs are negative. This pattern can arise in various situations. Firstly, false positivity for anti-HBc may occur, especially in low-prevalence populations. To address this issue, it is recommended to use state-of-the-art enzyme immunoassays and retest with another method.
Another reason for this serological pattern may be the inability of anti-HBs to reach detectable levels due to the formation of immune complexes resulting from the decline of HBsAg and anti-HBs during the window period of HBV infection. On the other hand, loss of anti-HBs may occur due to decreased immunity or the effects of treatments such as immunosuppressive drugs. Another situation that can lead to this serological profile involves mutations that can occur in the S-gene. HBsAg cases that cannot be detected in standard serological tests can emerge in mutations occurring in the “a” determinant (7).
In regions with low HBV prevalence, isolated anti-HBc positivity is observed in approximately 10 - 20% of individuals tested for HBV markers, indicating a community prevalence of 1 - 4% (11). However, in a study conducted in Korea with 17,677 participants, the estimated prevalence in the general population was reported to be 8.9% (14). In countries with high HBV infection prevalence, such as China, isolated anti-HBc positivity was found to be 12.3% (15). In both studies, the prevalence was higher in males and increased with age. In our study, isolated anti-HBc positivity was found to be 6%, which is lower than that reported in these studies. Additionally, when patients who were tested for HBsAg, anti-HBs, and anti-HBc were grouped according to age, the rate of isolated anti-HBc positivity was observed to increase with age. Therefore, the increase in the likelihood of viral reactivation through aging may lead to a higher incidence of isolated anti-HBc positivity in the older population. In addition, we believe that the inclusion of the HBV vaccine in the national vaccination plan in 1998 has contributed to the reduction in HBV infection risk in young individuals (16). The higher positivity rate for males in our study is consistent with findings from other studies (14, 15).
Among the patients who tested positive for HBV DNA, 77.3% were from the departments of gastroenterology, hematology, and internal medicine. Immunomodulatory therapies are commonly used in autoimmune diseases and conditions such as irritable bowel syndrome. Chemotherapeutic agents and immunosuppressive agents can also lead to HBV reactivation (17). Suppression of the immune system leads to increased HBV DNA replication and viral protein expression, resulting in tissue damage and initiating a process that can lead to death. Therefore, prophylaxis should be initiated for HBV DNA positivity in these patient groups, even if HBsAg is negative.
Berger et al. reported anti-HBc IgM positivity in patients with isolated anti-HBc positivity as 8.5% (18). When the tests were repeated with another device in the same study, this rate was found to be 2.1%. In our study, this rate was found to be 0.6%. Anti-HBc IgM may remain at detectable levels during acute infection (first six months) and acute exacerbations of chronic HBV infection. The fact that it does not stay in the blood at detectable levels for long may have contributed to the low rate of anti-HBc IgM observed in our study. We also believe that the characteristics of the methods and devices used, as well as the tested population, may have contributed to the differences in these rates between studies. Nevertheless, the presence of anti-HBc IgM in patients with isolated anti-HBc positivity is an indicator of viral replication, even if HBV DNA is below detectable levels (18). Therefore, testing for anti-HBc IgM in patients with isolated anti-HBc positivity can provide valuable information about HBV-related hepatitis.
The rate of HBV DNA positivity in the isolated anti-HBc pattern varies between 0 - 30% (2). In a study conducted by Rios-Ocampo et al. (19), this rate was found to be 1.9%, while Tramuto et al. (20) reported it as 5.2%, and Kishk et al. (21) identified it as 18.5%. In our study, this rate was 8.4%, which is consistent with the results of Tramuto et al. (20). Differences in the sensitivity of the devices and kits used in PCR testing, the primers used, and variations in PCR protocols can lead to differences in rates among studies. It is known that, particularly in occult HBV infection, the viral load is lower compared to asymptomatic carriers and chronic hepatitis patients, and the reason for this is considered to be suppressed viral replication (22). Thus, although the HBV DNA rate in our study was compatible with those found in other studies, we believe that the occult HBV infection rates in patients with isolated anti-HBc positivity are higher than these reported values.
Hepatitis B virus reactivation is a serious and often life-threatening complication that affects several risk groups, including patients receiving chemotherapy, immunosuppressive treatment, and organ transplant recipients. Reactivation can occur in patients who are HBsAg-positive or HBsAg-negative but anti-HBc-positive. This is because HBV DNA may remain under the viral detection limit. However, even when HBV DNA cannot be detected in the serum, HBV can persist in the liver. In patients with isolated anti-HBc, HBV reactivation can be identified by the emergence of HBsAg and/or HBV DNA (8, 12). Considering the strong connection between advanced age and the incidence of cancer, neglecting anti-HBc in HBV screening programs in endemic populations may disproportionately impact older individuals. The significantly higher prevalence of isolated anti-HBc positivity in older age groups in our study underscores the necessity of incorporating anti-HBc screening in this population.
Our study had several limitations. First, this was a retrospective study that only included patients for whom HBsAg, anti-HBs, and anti-HBc tests were ordered simultaneously. Retrospective studies generally carry a higher risk of bias compared to prospective studies, particularly due to potential data losses. Second, in Turkey, anti-HBc IgM tests are not routinely ordered in HBV screening, and it was not possible to access anti-HBc IgM results for all patients who tested positive for isolated anti-HBc.
Third, diagnosing occult HBV requires testing HBV DNA in the serum and/or liver biopsy samples. However, our study could not include HBV DNA test results obtained from liver biopsies. Fourth, HBsAg may not be detected by standard serological tests in cases with mutations in the “a” determinant. Our study lacked information on how many patients had undergone mutation testing. Finally, the results of this study are based on data collected from a single hospital. Future research involving a broader range of age groups and socioeconomic classes across multiple centers will help achieve more comprehensive and accurate results.
5.1. Conclusions
In conclusion, anti-HBc should be tested in addition to HBsAg and anti-HBs in the diagnosis of HBV. Anti-HBc IgM should be checked in isolated anti-HBc-positive patients to determine the window period, HBV DNA should be assessed to identify occult HBV infection, and mutation tests should be performed when necessary. The findings that most patients in our study were referred from departments related to internal medicine, that these patients were older, and that the rate of anti-HBc IgM positivity was low suggest that this pattern is more likely associated with antibody loss and/or viral reactivation occurring over time. Nonetheless, as HBV DNA tests in liver biopsies or mutation analyses were not conducted in this study, it is challenging to draw definitive conclusions.