1. Background
Pneumomediastinum (PM), the presence of air in the mediastinum, is a rare condition. Primary cases primarily occur in young men without trauma, surgery, or underlying medical conditions. Identified risk factors include smoking, recent respiratory infections, and interstitial lung disease (1-3). Air accumulation in the mediastinum results from alveolar rupture caused by a pressure gradient (4, 5). Secondary PM is rare but can be associated with viral respiratory infections (6-8). The classic triad of PM includes dyspnea, subcutaneous emphysema (SCE), and retrosternal pleuritic chest pain. Other symptoms may include coughing, neck pain, dysphagia, and odynophagia (9). Imaging is helpful for diagnosis, revealing characteristic features such as the Macklin effect and continuous diaphragm sign (10, 11).
During the COVID-19 pandemic, PM became more prevalent, particularly in patients receiving mechanical ventilation, especially those with acute respiratory distress syndrome (ARDS) (12). Positive pressure ventilation (PPV) poses a significant risk for PM in COVID-19 patients (13). Pneumomediastinum in COVID-19 patients is more common than in the general population, with intubated COVID-19 patients at a higher risk for both PM and SCE (14-16). The lung tissue infected by SARS-CoV-2 is more prone to alveolar rupture due to diffuse alveolar injury caused by cytokine release, leading to a higher likelihood of PM in COVID-19 patients (8). The onset of PM post-COVID-19 averages 19.6 days (7), with studies indicating a higher mortality rate in severe cases (8, 12).
Oxygen therapy is crucial for severe COVID-19 patients. However, invasive oxygenation increases the risk of complications due to barotrauma, such as PM and SCE. Oxygenation remains necessary for these patients, as these complications arise from COVID-19 and associated lung damage. Therefore, treatment approaches for PM in COVID-19 patients remain debatable. Positive pressure ventilation is generally contraindicated due to the risk of pulmonary barotrauma (17). Some studies suggest invasive interventions, such as chest drains, while others advocate for conservative management (18, 19). The high-flow nasal cannula has emerged as a newer method (20, 21).
2. Objectives
This study aims to assess the prevalence and outcomes of PM and SCE in COVID-19 patients, with a focus on identifying the primary risk factors associated with these complications.
3. Methods
3.1. Study Design
This retrospective cohort study was conducted on COVID-19 patients aged 18 - 80 years at Shohada-e Tajrish Hospital, Tehran, Iran, from April to December 2021. Eligible participants were those with confirmed COVID-19, based on a positive PCR test and a high-resolution chest CT scan. Patients with trauma, those lacking a positive PCR test or CT scan, or incomplete medical records were excluded. This exclusion criteria could introduce selection bias; however, we controlled for this bias by using a large study population. The primary aim of this study was to evaluate the presence of PM in COVID-19 patients. Given the notable incidence of SCE among PM patients, we also assessed SCE cases. Demographic and clinical data were extracted from patients' medical records. The high-resolution chest CT scans were evaluated by two radiologists who were blinded to the clinical data, and the severity of lung involvement was determined. A skilled radiologist re-evaluated the images of all included patients to assess the severity of lung involvement using the total severity score (TSS) (22). Control groups consisting of COVID-19 patients without PM or SCE complications were selected and matched by age, sex, and disease severity to enhance comparability with the PM and SCE cases.
3.2. Statistical Analysis
We performed statistical analysis using IBM SPSS Statistics (version 25.0). Categorical variables were presented as frequency (%), while quantitative variables were expressed as mean ± standard deviation (SD). To assess the relationships between categorical variables, we used the chi-square test and Fisher's exact test. For quantitative variables, we employed the independent t-test or its non-parametric equivalent. A logistic regression model was applied to control for confounding factors such as age, sex, and vital signs, and to evaluate the association between comorbidities, oxygenation, and ventilation with PM and SCE. A significance level of 0.05 was set for all statistical tests.
4. Results
From April to December 2021, 1 557 COVID-19 patients were identified, with 5.71% (89 patients) exhibiting PM. To assess PM, 89 age- and sex-matched control patients were included, resulting in a total of 178 participants with a mean age of 55.58 ± 15.58 years, of whom 71.9% (128) were male. Both groups exhibited similar lung involvement severity based on the TSS. The demographic characteristics of PM and control patients are shown in Table 1. Additionally, 4.04% (63 patients) had SCE (all with PM). To evaluate SCE, 63 control patients were added, resulting in a total of 126 participants in the study. Both control groups had COVID-19 without PM and SCE complications. The average hospitalization time was significantly longer in the PM group compared to the control group (18 days vs. 6 days; P < 0.0001). Clinical manifestations, particularly coughing and dyspnea, were significantly more common in the PM group. Clinical manifestations of the PM and control groups are shown in Table 2.
| Variables | Total | PM | Control | P-Value |
|---|---|---|---|---|
| Male | 128 (71.9) | 64 (50) | 64 (50) | 1.000 |
| Female | 50 (28.1) | 25 (50) | 25 (50) | |
| Age, y | 55.58 ± 15.58) | 55.96 ± 15.69 | 55.2 ± 15.55 | 0.748 |
Abbreviation: PM, pneumomediastinum.
a Values are expressed as mean ± SD or No. (%).
| Variables | PM | Control | P-Value |
|---|---|---|---|
| Fever | 17 (19.1) | 27 (30.3) | 0.082 |
| Fatigue | 16 (18) | 28 (30.8) | 0.054 |
| Coughing | 35 (39.8) | 20 (22.7) | 0.015 |
| Dyspnea | 54 (60.7) | 37 (42) | 0.013 |
| Myalgia | 11 (12.4) | 10 (11.8) | 0.998 |
Abbreviation: PM, pneumomediastinum.
a Values are expressed as or No. (%).
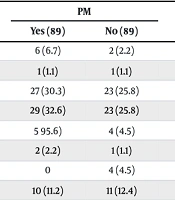
Comorbidities and risk factors related to PM and SCE are shown in Table 3. Although most of the comorbidities, especially respiratory conditions, were more common in the PM and SCE groups, these differences were not statistically significant. These conditions are known risk factors for severe COVID-19, while PM and SCE require diffuse alveolar damage to occur. Our study shows that none of the comorbidities have a significant relationship with the occurrence of PM and SCE. Analysis of oxygen therapy strategies revealed a significant association between PM and SCE and the use of Venturi masks, non-rebreather masks, non-invasive ventilation (NIV), and invasive mechanical ventilation (IMV). The relationship between different oxygenation methods and PM and SCE is shown in Table 4.
| Variables | PM | P-Value | SCE | P-Value | ||
|---|---|---|---|---|---|---|
| Yes (89) | No (89) | Yes (63) | No (63) | |||
| Asthma | 6 (6.7) | 2 (2.2) | 0.278 | 6 (9.5) | 1 (1.6) | 0.115 |
| Chronic obstructive pulmonary disease | 1 (1.1) | 1 (1.1) | 1.000 | 1 (1.6) | 0 | 0.315 |
| Diabetes | 27 (30.3) | 23 (25.8) | 0.505 | 20 (31.7) | 14 (22.2) | 0.316 |
| Hypertension | 29 (32.6) | 23 (25.8) | 0.323 | 17 (27) | 15 (23.8) | 0.838 |
| Hyperlipidemia | 5 95.6) | 4 (4.5) | 0.986 | 4 (6.3) | 2 (3.2) | 0.719 |
| Hyperthyroidism | 2 (2.2) | 1 (1.1) | 0.979 | 2 (3.2) | 1 (1.6) | 0.570 |
| Chronic kidney disease | 0 | 4 (4.5) | 0.141 | 0 | 4 (6.3) | 0.119 |
| Smoking | 10 (11.2) | 11 (12.4) | 0.816 | 8 (12.7) | 7 (11.1) | 0.738 |
Abbreviations: PM, pneumomediastinum; SCE, subcutaneous emphysema.
a Values are expressed as or No. (%).
| Variables | PM | P-Value | SCE | P-Value | ||
|---|---|---|---|---|---|---|
| Yes (89) | No (89) | Yes (63) | No (63) | |||
| Venturi and non-rebreather mask | 51 (57.3) | 38 (42.7) | < 0.0001 | 37 (58.7) | 21 (33.3) | 0.004 |
| NIV | 47 (52.8) | 6 (6.7) | < 0.0001 | 33 (52.4) | 3 (4.8) | < 0.0001 |
| IMV (intubation) | 37 (41.6) | 10 (11.2) | < 0.0001 | 29 (46.0) | 4 (6.3) | < 0.0001 |
Abbreviations: NIV, non-invasive ventilation; IMV, invasive mechanical ventilation; PM, pneumomediastinum; SCE, subcutaneous emphysema.
a Values are expressed as or No. (%).
After examining arterial blood gas and vital signs, no significant differences were found between the PM and SCE groups and the control groups. The arterial blood gas and vital signs for all patients are shown in Table 5. PM and SCE occurred, on average, 7.71 ± 6.64 and 8.34 ± 6.93 days after hospitalization, respectively. Following intubation, PM and SCE manifested at an average of 3.95 and 4.81 days, respectively. Treatment interventions included bilateral chest tubes in 23 PM and 17 SCE patients, while the remaining patients received oxygen therapy. Venous catheterization was performed in 23 PM and 14 SCE patients. Lung involvement severity, assessed by TSS in chest CT scans, did not differ significantly between the PM and SCE groups and their control groups. The chest CT scan findings and severity of lung involvement by TSS in the PM and SCE groups are shown in Table 6.
| Variables | PM | P-Value | SCE | P-Value | ||
|---|---|---|---|---|---|---|
| Yes (89) | No (89) | Yes (63) | No (63) | |||
| Arterial blood gas | ||||||
| HCO3 | 25.16 (4.88) | 26.19 (15.95) | 0.741 | 25.06 (4.13) | 26.87 (18.84) | 0.457 |
| PCO2 | 45.75 (12.39) | 46.82 (11.25) | 0.250 | 45.8 (11.82) | 47.43 (10.61) | 0.417 |
| pH | 7.36 (0.07) | 7.3 (0.14) | 0.101 | 7.35 (0.07) | 7.29 (0.16) | 0.111 |
| Vital signs | ||||||
| DBP | 76.61 (10.96) | 75.83 (12.56) | 0.661 | 76.41 (10.5) | 76.57 (11.89) | 0.937 |
| SBP | 122.74 (21.04) | 119.38 (20.28) | 0.280 | 122.58 (12.86) | 119.62 (20.6) | 0.436 |
| HR | 88.87 (18.83) | 91.37 (17.94) | 0.365 | 87.86 (19.65) | 91.67 (18.1) | 0.260 |
| RR | 21.7 (7.95) | 21.34 (6.03) | 0.739 | 22.08 (8.41) | 21.09 (5.61) | 0.439 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure; HR, heart rate; RR, respiratory rate; PM, pneumomediastinum; SCE, subcutaneous emphysema.
a Values are expressed as or mean (SD).
| Variables | PM (89) | SCE |
| Chest CT scan finding | ||
| GGO | 66 (74.2) | 43 (68.3) |
| Consolidation | 49 (55.1) | 34 (54) |
| White lung | 13 (14.6) | 12 (19) |
| Air bronchogram | 19 (21.3) | 11 (17.5) |
| Cavity | 1 (1.1) | 1 (1.6) |
| Fibrosis | 7 (7.9) | 2 (3.2) |
| Severity of lung involvement by TSS | 15.17 ± 6.36 | 15.52 ± 6.65 |
Abbreviations: PM, pneumomediastinum; SCE, subcutaneous emphysema; TSS, total severity score.
a Values are expressed as mean ± SD or No. (%).
Our study demonstrated a notable mortality rate in the PM and SCE groups, with the mortality rate being 43.8% (39) in the PM group and 47.6% (30) in the SCE group. After adjusting for possible confounders (age, sex, vital signs, and comorbidities), Venturi and non-rebreather masks, NIV, and IMV emerged as the main independent risk factors for PM in COVID-19 patients (P-value < 0.0001). In the logistic regression model, comorbidities were compared to the absence of comorbidities, and oxygenation and ventilation were compared to the absence of supplementary oxygen therapy. The association of all factors with the occurrence of PM is shown in Table 7.
| Variables | OR | CI 95% | P-Value |
|---|---|---|---|
| Comorbidities | |||
| Asthma | 3.374 | 0.644 - 17.76 | 0.150 |
| Chronic obstructive pulmonary disease | 1.003 | 0.060 - 16.04 | 0.990 |
| Diabetes | 1.232 | 0.625 - 2.430 | 0.547 |
| Hypertension | 1.419 | 0.687 - 2.931 | 0.344 |
| Hyperlipidemia | 1.223 | 0.308 - 4.856 | 0.775 |
| Hyperthyroidism | 1.987 | 0.173 - 22.807 | 0.581 |
| Oxygenation and ventilation | |||
| Venturi & non-rebreather masks | 3.075 | 1.658 - 5.705 | < 0.0001 |
| NIV | 16.941 | 6.545 - 43.851 | < 0.0001 |
| IMV | 5.703 | 2.595 - 12.533 | < 0.0001 |
| Arterial blood gas | |||
| HCO3 | 0.992 | 0.964 - 1.021 | 0.594 |
| PCO2 | 0.992 | 0.967 - 1.018 | 0.543 |
| Vital signs | |||
| SBP | 1.008 | 0.993 - 1.023 | 0.303 |
| DBP | 1.005 | 0.979 - 1.031 | 0.705 |
| Heart rate | 0.993 | 0.976 - 1.009 | 0.386 |
| Respiratory rate | 1.008 | 0.966 - 1.015 | 0.721 |
| SpO2 | 1.014 | 0.998 - 1.031 | 0.083 |
Abbreviations: NIV, non-invasive ventilation; IMV, invasive mechanical ventilation; DBP, diastolic blood pressure; SBP, systolic blood pressure; OR, odds ratio.
5. Discussion
The findings of our study provide valuable insights into the occurrence, clinical characteristics, and outcomes of PM and SCE in COVID-19 patients. We observed a notable incidence of PM (5.71%) and SCE (4.04%) among the 1 557 COVID-19 patients identified from April to December 2021. The inclusion of age, sex, and severity-matched control groups allowed us to make a comprehensive comparison. The prevalence rates of PM and SCE reported in various studies differ from our findings. Manna et al. reported a higher PM rate of 91%, while Muley et al. observed rates of 14.5%, Elsaaran et al. reported 14.8%, and Kangas-Dick et al. found a rate of 10% (18, 23-25). Additionally, the prevalence of SCE was noted to be 36% in the Manna et al. (23) study and 13.6% in the Lemmers et al. study (26). These variations could be due to differences in patient selection, inclusion/exclusion criteria, population size, definitions of conditions/complications/outcomes, and study types and settings.
Our study revealed that patients with PM had a significantly longer average hospitalization time compared to the control group (18 days vs. 6 days; P < 0.0001). This extended hospital stay in the PM group may reflect the complexity and severity of their clinical course. Clinical manifestations, such as coughing and dyspnea, were significantly more prevalent in the PM group, indicating the potential impact of PM on respiratory symptoms. In our study, most of the comorbidities were more common in the PM and SCE groups, particularly respiratory conditions like asthma and COPD. This finding was predictable due to their role in severe cases of COVID-19. However, these comorbidities and risk factors did not show a significant association with the occurrence of PM and SCE. Studies by Buyukkarabacak et al. and Manna et al. have confirmed these findings (23, 27), while Raykar et al. concluded that obesity and asthma are associated with a poor prognosis in PM patients (28).
Analysis of oxygen therapy highlighted a significant association between PM and SCE and the use of Venturi masks, non-rebreather masks, NIV, and IMV, confirming the results of other studies (25, 27, 29). Oxygen therapy is necessary for COVID-19 patients, including those with complications such as PM and SCE. However, the association between oxygen therapy in COVID-19 and the occurrence of these complications underscores the importance of considering oxygenation and ventilation strategies in managing COVID-19 patients at risk of developing PM and SCE. Further large-scale studies are required to determine the optimal strategy for oxygen therapy in patients with PM and SCE.
No significant differences in arterial blood gas and vital signs were observed. PM and SCE manifested, on average, 7.71 and 8.34 days after hospitalization, respectively, and 3.95 and 4.81 days after intubation in patients receiving IMV. Variability in reported timelines for the occurrence of complications exists across studies. Regarding PM after hospitalization, Al-Dorzi et al. found an average duration of 4 days, whereas Raykar et al. reported 17.3 days, and Abdelghany et al. observed 14 days (16, 28, 29). For SCE after hospitalization, Manna et al. and Hayrabedian et al. reported average intervals of 13.3 and 8 days, respectively (23, 30).
These divergent timelines highlight the nuanced nature of complication onset, likely influenced by factors such as patient characteristics, study methodologies, and specific clinical contexts. The mortality rates were 43.8% in the PM group and 47.6% in the SCE group. After adjusting for age and sex, our analysis identified the use of Venturi and non-rebreather masks, IMV, and NIV as significant risk factors for the occurrence of PM in COVID-19 patients, underscoring the importance of careful consideration in oxygenation and ventilation strategies.
5.1. Conclusions
Generally, COVID-19 leads to a cytokine storm, causing inflammation and complications in multiple organs, predominantly in the lungs. The affected lungs become brittle and more prone to alveolar damage, which may lead to PM and SCE. In our study, the use of Venturi and non-rebreather masks, NIV, and IMV in COVID-19 patients were identified as the most significant risk factors for PM and SCE. This reflects the higher susceptibility of lungs in COVID-19 to severe damage from oxygenation and ventilation. Therefore, in COVID-19 patients requiring mechanical ventilation in intensive care units, cautious and closely controlled oxygenation and ventilation, or alternative oxygenation methods such as high-flow nasal cannula, should be considered to reduce the risk of barotrauma leading to PM and SCE.