1. Background
Systemic inflammatory response syndrome (SIRS) is widely recommended as a screening tool to identify pediatric patients at risk of septic shock who may require resuscitative care. As part of the formal definition of sepsis, SIRS has been effectively utilized in the Pediatric Surviving Sepsis campaign to activate pediatric sepsis protocols, despite some concerns regarding its potential for over-sensitivity in pediatric emergency settings (1, 2). Systemic inflammatory response syndrome encompasses systemic inflammatory responses arising from various severe conditions, including infection, trauma, hypoxia-ischemia, and shock. Respiratory infections frequently lead to SIRS and sepsis, although many cases of clinical deterioration lack bacterial involvement, suggesting that viruses may also play a significant role (3-5).
The emergence of coronavirus disease 2019 (COVID-19) in China and globally has renewed attention on viral sepsis among clinicians and researchers (6). While advancements have been made in understanding the pathophysiology of viral sepsis, its distinctions from bacterial sepsis remain poorly understood. Moreover, diagnostic methods to reliably differentiate between viral and bacterial sepsis—especially in the early stages—are limited.
Common respiratory viruses include human rhinovirus (HRV), influenza viruses (FluA/B), respiratory syncytial virus (RSV), human parainfluenza viruses (HPIV), human coronaviruses (HCoV), human adenovirus (HAdV), human metapneumovirus (hMPV), and human bocavirus (HBoV). Literature on respiratory viruses associated with SIRS in pediatric patients is notably sparse, primarily consisting of isolated case reports (7, 8). These cases provide valuable but fragmented insights into the impact of respiratory viruses on severe systemic conditions in children. However, the available data remains insufficient to form more generalized conclusions or to guide comprehensive clinical practices.
2. Objectives
This research aims to examine the relationship between respiratory viral infections and SIRS in pediatric patients, focusing on the prevalence of these infections, associated clinical and demographic characteristics, and the rate of septic shock associated with various respiratory viruses.
3. Methods
3.1. Study Population
A retrospective analysis was conducted on hospitalized pediatric patients who met the clinical criteria for both respiratory infections and SIRS from January 2022 to December 2023. Cases were excluded based on the following criteria: (1) neonates and preterm infants; (2) underlying chronic respiratory or other systemic diseases; (3) congenital or genetic disorders affecting respiratory or other systems; (4) co-infections with other pathogens (e.g., bacteria, fungi, mycoplasma, or chlamydia) or concurrent infections at other anatomical sites; (5) insufficient clinical information. The study was approved by the Maternal and Child Health Hospital of Hubei province, with informed consent waived due to its retrospective nature and use of anonymized clinical data.
3.2. Definitions
Acute respiratory tract infections were classified as upper respiratory infection, bronchitis, or pneumonia based on clinical symptoms, physical examination findings, and chest X-ray results where available. The criteria for pediatric SIRS and septic shock were based on the 2005 Expert Consensus and the 2012 International Guidelines, respectively (1, 2).
3.3. Laboratory Tests
Nasopharyngeal secretions or alveolar lavage fluid collected within 24 hours of admission were analyzed. Respiratory virus detection was conducted using commercial qualitative PCR capillary electrophoresis kits (Ningbo Haiers Gene Technology Co., Ltd., China), targeting FluA/B, HRV, RSV, HPIV, HCoV, HAdV, hMPV, HBoV, Mycoplasma pneumoniae, and Chlamydia. Microbial culture and identification were conducted within 24 hours of admission, following the hospital’s standard diagnostic procedures. Procalcitonin levels in serum were measured via enzyme-linked fluorescent immunoassay using the Vidas BRAHMS PCT kit (bioMérieux, Marcy l’Etoile, France).
3.4. Statistical Analyses
Statistical analyses were performed using SPSS version 21.0 software (SPSS, Inc., Chicago, IL, USA). Comparisons of frequencies between groups were analyzed using Chi-squared tests, with a P-value of less than 0.05 considered statistically significant.
4. Results
4.1. Study Profile
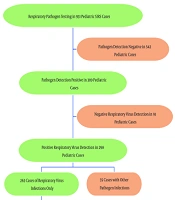
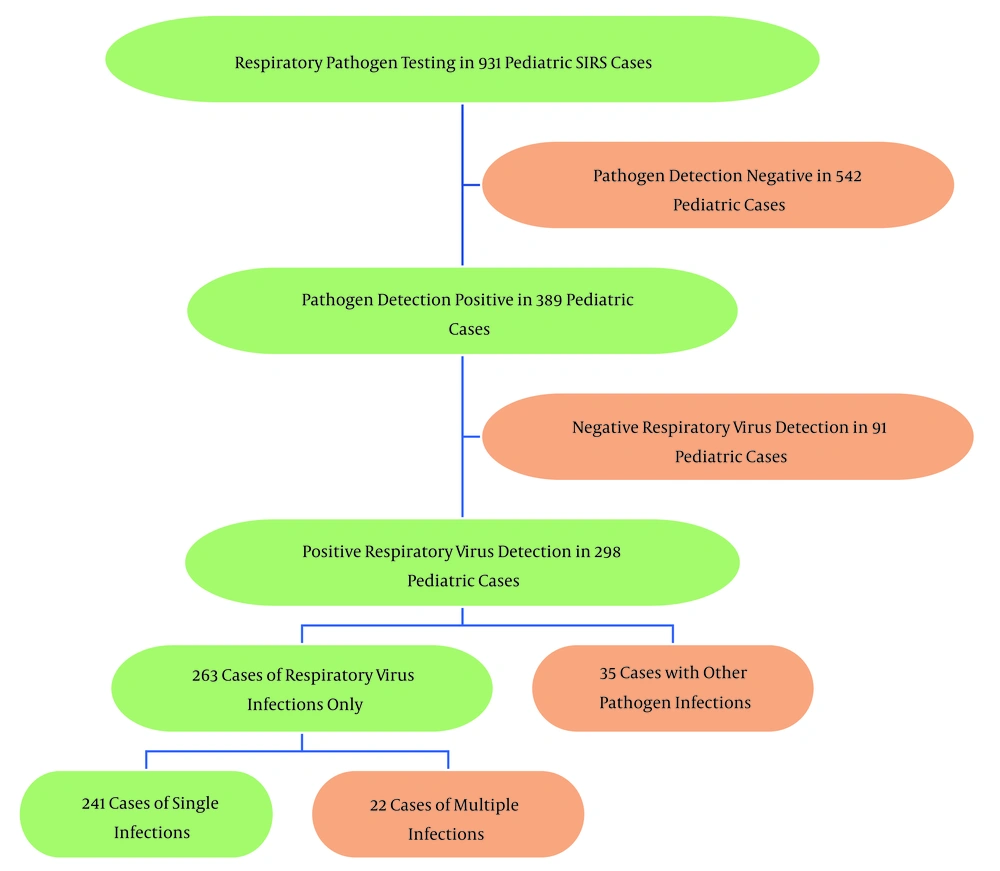
Between January 2022 and December 2023, a total of 931 pediatric patients with SIRS were tested for respiratory viruses and/or other pathogens. Among these, 542 cases (58.2%) were negative for pathogens. Out of the 389 cases that tested positive for pathogens, 298 (32.0%, 298/931) were positive for respiratory viruses, with 263 (28.2%, 263/931) being attributed solely to respiratory viruses. Detailed pathogen detection results are shown in Figure 1.
4.2. Pathogen Detection in Pediatric Respiratory Infections Associated with Systemic Inflammatory Response Syndrome
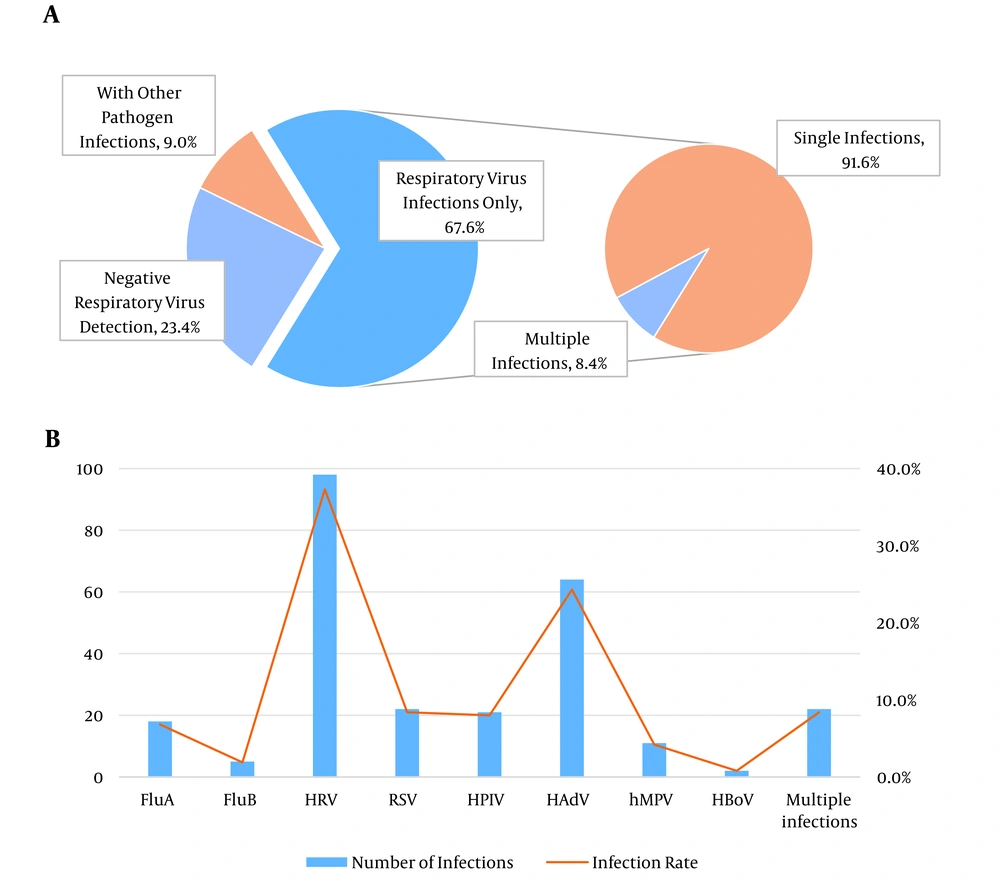
In the 389 cases with positive pathogen detection, 91 children tested negative for respiratory viruses, representing 23.4% (91/389) of the cases. Of the remaining 298 cases with positive respiratory virus detection, 35 (9.0%, 35/389) involved co-infections with other pathogens, and 263 (67.6%, 263/389) were solely due to respiratory virus infections (Figure 2A).
4.3. Detection Rates of Respiratory Viruses in Children with Systemic Inflammatory Response Syndrome
As illustrated in Figure 2B, among the 263 cases of respiratory virus infections, HRV had the highest detection rate at 37.3% (98/263), followed by HAdV at 24.3% (64/263). Other notable detection rates included FluA, RSV, HPIV, and mixed infections, all with rates above 5%.
4.4. Demographics of Pediatric Patients with Systemic Inflammatory Response Syndrome Potentially Due to Respiratory Viral Infections
Table 1 presents the demographic and seasonal distribution of the 263 pediatric patients with SIRS potentially caused by respiratory viral infections. Among these patients, 51.0% were male. Ages ranged from 1 month to 13 years, with a mean age of 2.91 ± 2.26 years and a median of 3 years. The majority (69.2%) were aged between 1 and 5 years, while 17.8% were under 1 year and 13.0% were aged 6 years or older. Seasonally, the highest incidence occurred in spring (39.5%), followed by summer (25.5%), winter (17.9%), and autumn (17.1%).
| Parameter | Values |
|---|---|
| Sex | |
| Male | |
| No. (%) | 134 (51.0) |
| Age | |
| < 1 | |
| Month (median, range) | 6 (1 - 11) |
| Mean ± standard deviation | 5.70 ± 3.078 |
| No. (%) | 47 (17.8) |
| 1 - 5 | |
| Year (median, range) | 3 (1 - 5) |
| Mean ± standard deviation | 2.73 ± 1.204 |
| No. (%) | 182 (69.2) |
| ≥ 6 | |
| Year (median, range) | 6 (6 - 13) |
| Mean ± standard deviation | 7.26 ± 2.093 |
| No. (%) | 34 (13.0) |
| Season | |
| Spring (March, April and May) | 104 (39.5) |
| Summer (June, July and August) | 67 (25.5) |
| Autumn (September, October, and November) | 45 (17.1) |
| Winter (December, January, and February) | 47 (17.9) |
Demographics of 263 Pediatric Patients with Systemic Inflammatory Response Syndrome Potentially Due to Respiratory Viral Infections.
4.5. Clinical and Demographic Data of Pediatric Systemic Inflammatory Response Syndrome Patients with Respiratory Viral Infections
Table 2 presents the primary clinical characteristics of 263 pediatric patients with SIRS potentially attributed to respiratory viral infections. Fever was prevalent, affecting over 80% of cases for most viruses, with HBoV and hMPV showing the highest incidence at 100%. Increased respiratory rates were notably common, particularly in infections caused by HAdV (92.2%) and hMPV (81.8%). Tachycardia was frequently observed in cases of RSV (90.9%) and hMPV (72.7%). Approximately 20% of patients had normal plasma procalcitonin levels, with no significant elevations detected.
| Parameter | HRV (n = 98) | FluA (n = 18) | FluB (n = 5) | RSV (n = 22) | HAdV (n = 64) | HPIV (n = 21) | hMPV (n = 11) | HBoV (n = 2) | Coinfections (n = 22) |
|---|---|---|---|---|---|---|---|---|---|
| Upper respiratory infection | 53 (54.1) | 10 (55.6)) | 2 (40.0) | 7 (31.8) | 34 (53.1) | 4 (19.0) | 2 (18.2) | 1 (50.0) | 7 (31.8) |
| Tracheitis/bronchitis | 17 (17.3) | 2 (11.1)) | 1 (20.0) | 3 (13.6) | 11 (17.2) | 8 (38.1) | 1 (9.0) | 1 (50.0) | 4 (18.2) |
| Pneumonia/bronchopneumonia | 28 (28.6) | 6 (33.3) | 2 (40.0) | 12 (54.5) | 19 (29.7) | 9 (42.9) | 8 (72.7) | 0 (0) | 11 (50.0) |
| Fever (> 38.5°C) | 79 (80.6) | 15 (83.3) | 4 (80.0) | 16 (72.7) | 61 (95.3) | 17 (81.0) | 11 (100.0) | 2 (100.0) | 20 (90.9) |
| Tachycardia | 59 (60.2) | 10 (55.6) | 3 (60.0) | 20 (90.9) | 37 (57.8) | 15 (71.4) | 8 (72.7) | 0 (0) | 8 (36.4) |
| Mean respiratory rate>2 SD above normal for age | 56 (57.1) | 10 (55.6) | 3 (60.0) | 15 (68.2) | 59 (92.2) | 13 (61.9) | 9 (81.8) | 0 (0) | 16 (72.7) |
| Leukocyte count elevated or depressed for age | 63 (64.3) | 8 (44.4) | 3 (60.0) | 10 (45.5) | 34 (53.1) | 10 (47.6) | 3 (27.3) | 2 (100.0) | 11 (50.0) |
| Plasma procalcitonin < 0.05 ng/mL | 21 (21.4) | 6 (33.3) | 1 (20.0) | 5 (22.7) | 15 (23.4) | 6 (28.6) | 2 (18.2) | 0 (0) | 6 (27.3) |
Clinical and Demographic Data of Pediatric Systemic Inflammatory Response Syndrome Patients with Respiratory Viral Infections a
4.6. Incidence of Septic Shock Caused by Various Viral Infections
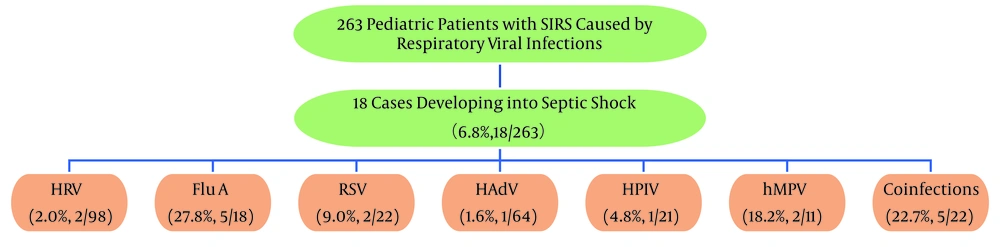
Among the 263 children with SIRS due to respiratory viral infections, 6.8% (18/263) progressed to septic shock. The highest rates of septic shock occurred with FluA (27.8%, 5/18), hMPV (18.2%, 2/11), and coinfections (22.7%, 5/22). For details on septic shock rates associated with other viral infections, refer to Figure 3. The differences in septic shock rates between various viral infections were statistically significant (P < 0.001).
5. Discussion
In this study of 931 children with SIRS, respiratory viruses were detected in 32.0% of cases, increasing to 67.6% among those with positive pathogen tests. A prospective study found that respiratory viral infections were identified in 17% of pediatric sepsis cases and 48.2% of adult sepsis cases (3), highlighting the significant role respiratory viruses play in pediatric SIRS and indicating they are a major factor in its onsetindings underscore the need for comprehensive viral screening in pediatric intensive care units and the importance of developing targeted antiviral therapies and supportive measures for managing pediatric SIRS.
Respiratory viruses, including Flu A/B, HRV, RSV, HPIV, HAdV, hMPV, and HBoV, can all lead to severe illness in children (9-11). In pehe detection rates of HRV, HAdV, Flu A, RSV, HPIV, and mixed infections were each above 5%, consistent with previous research findings (3, 12). However, a positive vira confirm viral sepsis or SIRS; clinicians must consider potential co-infections with other undetected pathogens or the possibility of false positives. Thorough clinical assessment—including symptoms, lab results, and imaging—is essential for accurate diagnosis and management.
Respiratory viruses often display distinct seasonal patterns (13, 14). Our study showed that HRV peaks in Wuhan from March to July, while HAdV peaks from May to August. In this study, we observed that SIRS detection rates in children were highest for HRV and HAdV, aligning with the majority of virus-related SIRS cases occurring in spring and summer. Our findings also indicated that 15% to 30% of pediatric patients with respiratory viral infections did not exhibit elevated procalcitonin levels, and even when elevated, the increase was often minimal. Studies have shown that markedly high procalcitonin levels are typically associated with bacterial rather than viral infections (15, 16). Furthermore, a pstrated that patients with sepsis and negative cultures had significantly lower procalcitonin levels (17). Consequently, if procalcitonin levelslightly elevated or normal, a viral infection should be strongly considered.
This finding highlights the complex relationship between viral pathogens and disease severity in pediatric SIRS. Although HRV and HAdV were more prevalent, the higher risk of septic shock associated with FluA, hMPV, and co-infections suggests these viruses may trigger more severe systemic responses. Previous studies have shown that FluA infections are associated with severe sepsis, with adults experiencing influenza infections at a higher risk of septic shock compared to those with HRV infections (18-20). Additional research has confirmed that viral conditions and elevate the risk of shock. However, more studies are needed to further clarify the relationship between co-infections and septic shock (10, 21, 22).
These findings underscore the importance of virus-specific approaches in musative virus may significantly impact the risk of progression to septic shock. Future research should aim to elucidate the mechanisms by which different respiratory viruses interact with the host immune system, providing insight into more effective, targeted therapeutic interventions. Our study has two main limitations. First, as a retrospective analysis with a limited scope for pathogen detection in pediatric patients, certain infections may have been missed, or the pathogens identified may not be the actual causes of the illnesses. Second, a substantial number of febrile children had received antibiotics prior to admission, which may have contributed to negative results in pathogen detection and blood cultures.
5.1. Conclusions
In conclusion, many children hospitalized with respiratory viral infections are at risk of developing SIRS, with some cases potentially progressing to septic shock. For cases where SIRS is present and no bacterial, mycoplasma, or fungal pathogens are detected, it is essential to consider viral infections as a possible cause and conduct appropriate viral pathogen testing.