1. Background
Psittacosis, commonly referred to as parrot fever or ornithosis, is an infectious bacterial disease caused by Chlamydia psittaci (1). This bacterium is an obligate intracellular gram-negative organism. Psittacosis can be transmitted to humans through the respiratory tract via air or aerosols, causing a type of pneumonia known as atypical community-acquired pneumonia (CAP). It can also invade human skin, mucous membranes, and the digestive tract via exposure to contaminated bird droppings (1). During the 1930s, parrot fever was widespread in 12 nations and directly contributed to the formation of the National Institutes of Health (2). While there have been limited instances of person-to-person transmission of C. psittaci (3-6), sporadic outbreaks have occurred in various locations over the past few decades (7-10). Studies have suggested that the prevalence of C. psittaci may be at least 1% of cases of C. psittaci pneumonia (11). Diagnosing C. psittaci pneumonia in humans can be challenging, leading to misdiagnosis and underdiagnosis. Unlike traditional microbiological diagnostic methods, C. psittaci pneumonia often goes unrecognized.
Chlamydia psittaci primarily triggers respiratory infections in humans, showcasing diverse clinical manifestations. Following initial replication in the epithelial cells and macrophages of the respiratory system, the bacteria can disseminate throughout the body, impacting various organs. The intensity of C. psittaci pneumonia can vary from mild flu-like symptoms to severe, life-threatening pneumonia (1). Due to the diverse clinical features of C. psittaci pneumonia and minimal human-to-human transmission, despite previous reports suggesting that C. psittaci pneumonia can be diagnosed by serology, C. psittaci isolation, and molecular assays (12), its nonspecific symptoms and limitations of traditional testing methods (9) make it easy to be misdiagnosed, especially when influenza and COVID-19 cases overlap (6). For example, the initial clinical presentation of C. psittaci pneumonia includes fever, cough, dyspnea, malaise, headache, and myalgia, similar to COVID-19. The most common CT images in patients with C. psittaci pneumonia include solid lesions and pleural effusion, also similar to COVID-19 (13, 14).
Similarly, viral pneumonia, caused by various respiratory viruses, can present with similar clinical features, further complicating the diagnostic process. Early and accurate identification of the underlying etiology is crucial for appropriate treatment and infection control measures. The treatment options for C. psittaci pneumonia and viral pneumonia are different. Chlamydia psittaci pneumonia is mainly treated with antibiotics such as doxycycline and moxifloxacin, whereas viral pneumonia requires the use of antiviral drugs such as ribavirin and oseltamivir phosphate. Accurate diagnosis will help to choose the appropriate treatment plan and improve the effectiveness of treatment. In addition, the transmission modes of C. psittaci pneumonia and viral pneumonia differ. Identifying the pathogen type aids in implementing specific preventive strategies to prevent the spread of the disease. Therefore, the development of reliable biomarkers to differentiate C. psittaci from viral pneumonia is of great clinical importance (15).
Interferon-gamma (IFN-γ) is a signaling protein generated by activated T-cells and natural killer cells when combating intracellular pathogens such as C. psittaci and specific viral infections (16). Prior research indicates that IFN-γ contributes to the immune defense against these infections (17). However, the diagnostic potential of IFN-γ in differentiating C. psittaci from viral pneumonia remains largely unexplored. Next-generation sequencing (NGS) is an efficient, high-throughput method for the simultaneous identification of different pathogens in the clinic, increasing the detection of rare and unexpected pathogens (18). Thus, NGS can provide relatively rapid diagnosis to expedite antibiotic therapy and improve patient prognosis. In recent years, NGS has been used for confirmatory diagnosis of C. psittaci infection, but has been limited to a few case reports and a small number of case series (19, 20). Therefore, the present study collected clinical data describing NGS-diagnosed confirmed cases of C. psittaci pneumonia and viral pneumonia and retrospectively analyzed the differences in clinical symptoms, serology, and imaging between the two groups.
2. Objectives
The aim was to explore the potential role of IFN-γ as a biomarker for distinguishing between C. psittaci pneumonia and viral pneumonia, and to provide guidance for early identification and diagnosis of C. psittaci pneumonia in clinical practice.
3. Methods
3.1. Study Design and Participants
In this retrospective multicenter study, 22 patients diagnosed with C. psittaci pneumonia by NGS at Nanjing First Hospital between February 2018 and February 2023 were included as the C. psittaci pneumonia group. Additionally, 22 cases of viral pneumonia diagnosed by serology at this hospital during the same period were selected as the viral pneumonia group.
3.1.1. Case Inclusion Criteria
(1) All patients met the diagnostic standards outlined in the 2016 guidelines for the diagnosis and management of community-acquired pneumonia in Chinese adults by the respiratory branch of the Chinese Medical Association.
(2) Diagnostic criteria for C. psittaci pneumonia conformed to the clinical diagnostic criteria for CAP; NGS detected DNA fragments specific for C. psittaci; a positive result was obtained by C. psittaci-specific PCR, and negative results were shown for all routine pathogenic pathogens on sputum, blood, or alveolar lavage samples; no other causative microorganisms were identified by metagenomic NGS (mNGS) or serologic testing. Clinical symptoms included fever, cough, sputum, and chest pain, and imaging changes showed significant pulmonary infiltrates.
(3) Diagnostic criteria for viral pneumonia were based on the diagnostic guidelines outlined in the 2010 Expert Consensus on the Diagnosis and Treatment of Adult Pneumonia by the Respiratory Department of the Chinese Medical Association.
3.1.2. Case Exclusion Criteria
(1) Patients with severe hepatic or renal insufficiency, blood system disorders, or immune system diseases were excluded. Individuals with severe hepatic or renal insufficiency may not be able to metabolize or excrete the drug properly and may have unstable health conditions that increase the risk during the study. Those with hematologic disorders may affect the detection and analysis of blood-related markers in the study. Those with immune system disorders may affect their immune response to C. psittaci or viral infections.
(2) Incomplete clinical data resulted in exclusion from the study.
3.2. Data Collection
Data were extracted from electronic health records, encompassing age, gender, duration of hospitalization, clinical symptoms, epidemiological information, concurrent medical conditions, laboratory assessments, and radiological findings. Laboratory findings included total white blood cell (WBC) count, neutrophil and lymphocyte percentages, procalcitonin (PCT), IFN-γ, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). Fasting venous blood was collected in 5 mL within 24 hours before treatment and centrifuged at 3000 rpm for 5 minutes. The WBC, neutrophil, and lymphocyte counts were determined using a fully automated biochemistry analyzer (Mindray, Shenzhen, China, model 7500), and their percentages were calculated. Serum PCT and IFN-γ levels were measured by enzyme-linked immunosorbent assay (ELISA) using a PCT kit (Amyjet Scientific, Wuhan, China) and an IFN-γ kit (Y-S Biotechnology, Shanghai, China). The IL-6 level was measured by radioimmunoassay. Lung imaging, detailing lesion locations and characteristics, was evaluated by two experienced radiologists.
3.3. Statistical Analysis
Statistical analyses were conducted using appropriate software. Continuous variables were represented as mean with standard deviation (SD), while categorical variables were depicted as frequencies and percentages. The independent t-test or Mann-Whitney U test was applied for comparing continuous variables between groups, depending on their distribution. The diagnostic utility of inflammatory markers in differentiating C. psittaci pneumonia from viral pneumonia was assessed by area under the curve (AUC) analysis of receiver operating characteristic (ROC) curves. Positive predictive value (PPV) and negative predictive value (NPV) were calculated based on sensitivity and specificity. Samples lacking data were eliminated, and the analysis focused on complete data. Samples with indeterminate results were not included in the pathologic diagnostic information for the study, resulting in the removal of those with physician-annotated indeterminate grades. Statistical significance was denoted by a P-value of less than 0.05.
4. Results
4.1. Clinical Information of Patients
Clinical characteristics are summarized in Table 1. The study encompassed a cohort of 44 participants, comprising 22 individuals diagnosed with C. psittaci pneumonia and another 22 diagnosed with viral pneumonia. The mean age of participants in the C. psittaci pneumonia group was 63.6 ± 11.8 years, and 54.5% were male. The average age of individuals in the viral pneumonia group was 77.7 years, with a standard deviation of 11.4 years, and 63.6% were male. Common symptoms observed in both groups included fever, cough, and sputum production.
| Variables | Total (n = 44) | Chlamydia psittaci Pneumonia (n = 22) | Virtal Pneumonia (n = 22) | P-Value b |
|---|---|---|---|---|
| Age (y) | 70.7 ± 13.5 | 63.6 ± 11.8 | 77.7 ± 11.4 | < 0.001 |
| Male | 26 (59.1) | 12 (54.5) | 14 (63.6) | 0.540 |
| Fever | 39 (88.6) | 22 (100) | 17 (77.3) | 0.048 |
| Cough | 42 (95.6) | 21 (95.5) | 21 (95.5) | 1.000 |
| Sputum | 28 (63.6) | 7 (31.8) | 21 (95.5) | < 0.001 |
| Nervous system symptoms | 11 (25.0) | 10 (45.5) | 1 (4.5) | 0.002 |
| Lesions in left lung | 33 (75.0) | 11 (50.0) | 22 (100) | < 0.001 |
| Lesions in right lung | 37 (84.1) | 16 (72.7) | 21 (95.5) | 0.039 |
| Lesions in bilateral lungs | 26 (59.1) | 5 (22.7) | 21 (95.5) | < 0.001 |
| Hospital (d) | 14.4 ± 6.4 | 13.4 ± 4.0 | 15.4 ± 8.2 | 0.311 |
| The maximum body temperature (℃) | 38.7 ± 0.96 | 39.4 ± 0.44 | 38.1 ± 0.91 | < 0.001 |
| White blood cell count (× 109/L) | 7.66 ± 3.36 | 7.30 ± 2.72 | 8.02 ± 3.94 | 0.486 |
| Neutrophil percentage (%) | 80.3 ± 10.8 | 84.4 ± 7.9 | 76.3 ± 11.9 | 0.011 |
| Lymphocyte percentage (%) | 12.6 ± 7.6 | 9.9 ± 5.7 | 15.2 ± 8.4 | 0.019 |
| IFN-γ | 8.97 (4.29 - 62.24) | 60.93 (7.67 - 368.46) | 5.75 (4.02 - 9.56) | 0.002 |
| IL-6 | 27.99 (9.78 - 128.31) | 94.86 (28.03 - 188.51) | 11.91 (6.12 - 27.12) | < 0.001 |
| TNF-α | 1.74 (1.54 - 2.28) | 1.74 (1.51 - 2.36) | 1.74 (1.50 - 2.02) | 0.820 |
| PCT | 0.41 (0.07 - 1.17) | 0.94 (0.20 - 2.76) | 0.15 (0.06 - 0.37) | 0.005 |
Clinical Baseline Characteristics, Laboratory Results and Imaging Results of Psittacosis and Viral Pneumonia Groups a
No significant gender differences were observed between the two groups (P > 0.05). However, the C. psittaci pneumonia group exhibited a significantly younger age compared to the viral pneumonia group (P < 0.001), along with higher maximum body temperature readings (P < 0.001). Although there were no notable variations in cough prevalence between the groups (P > 0.05), the proportion of expectoration was notably lower in the C. psittaci pneumonia group compared to the viral pneumonia group, showing statistical significance (P < 0.001). Patients with C. psittaci pneumonia demonstrated a higher incidence of nervous system symptoms (45.5%) in comparison to those with viral pneumonia (P < 0.002).
4.2. Analysis of Laboratory and Imaging Data
There were no statistically significant disparities in white blood cell count and TNF-α levels between the two patient groups (P > 0.05). However, the C. psittaci pneumonia group exhibited higher neutrophil percentages and IFN-γ levels compared to the viral pneumonia group, while displaying lower lymphocyte percentages and PCT levels (P < 0.05).
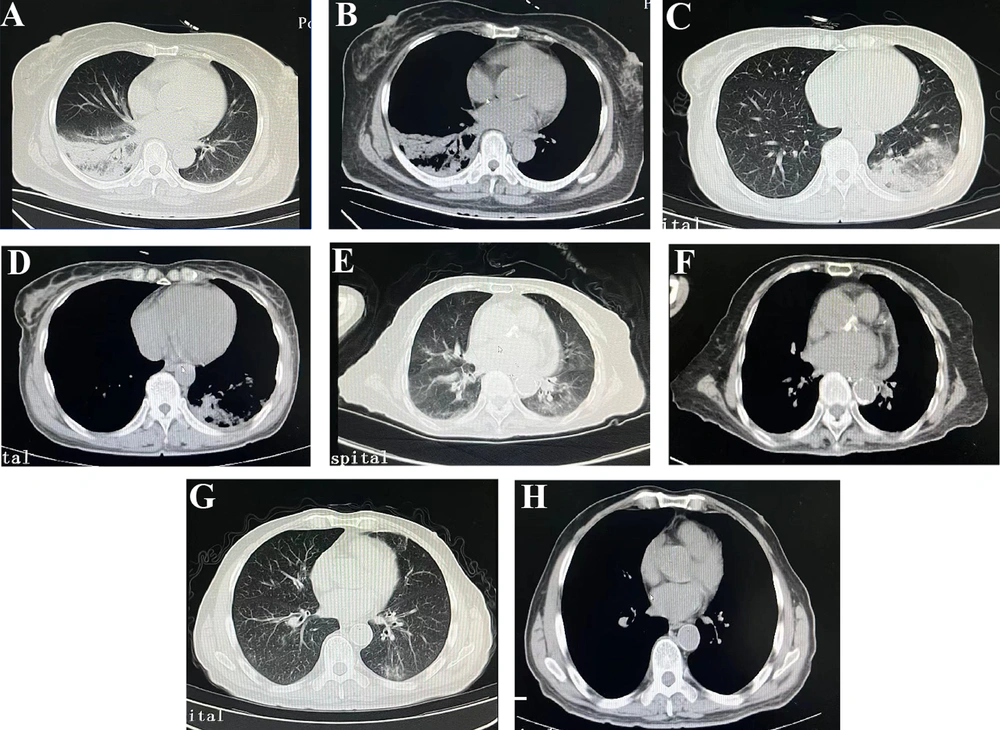
Chest CT findings of four patients showed varying degrees of lesions. Analysis of chest CT scans revealed imaging changes in patients with C. psittaci pneumonia, with right, left, and bilateral lung involvement in 50%, 72.7%, and 22.7%, respectively. Unilateral, non-uniformly dense lung shadows, appearing as large or mass-like formations with blurred edges and early exudative changes, were observed in 77.27% of patients with C. psittaci pneumonia. Solid changes with air-filled bronchial shadows became predominant in the later stages. The most common imaging feature on chest CT scan at admission was unilateral solid lesions in the lungs. Imaging manifestations in patients with viral pneumonia found 100%, 95.5%, and 95.5% of patients with right, left, and bilateral lung involvement, respectively. Chest CT scans demonstrating bilateral lung lesions were more common in patients with viral pneumonia than in patients with C. psittaci pneumonia (95.5% vs 22.7%, P < 0.001) (Table 1 and Figure 1).
Chest computed tomography images of patients with Chlamydia psittaci pneumonia group or viral pneumonia group; A and B, a 64-year-old woman with C. psittaci pneumonia exhibiting unilateral right lung consolidation; C and D, a 63-year-old woman with C. psittaci pneumonia exhibiting unilateral left lung consolidation; E and F, a 88-year-old woman with viral pneumonia exhibiting bilateral lung consolidation; G and H, a 69-year-old man with viral pneumonia exhibiting consolidation distributed diffusely in both lungs.
4.3. Receiver Operating Characteristic Curve Analysis
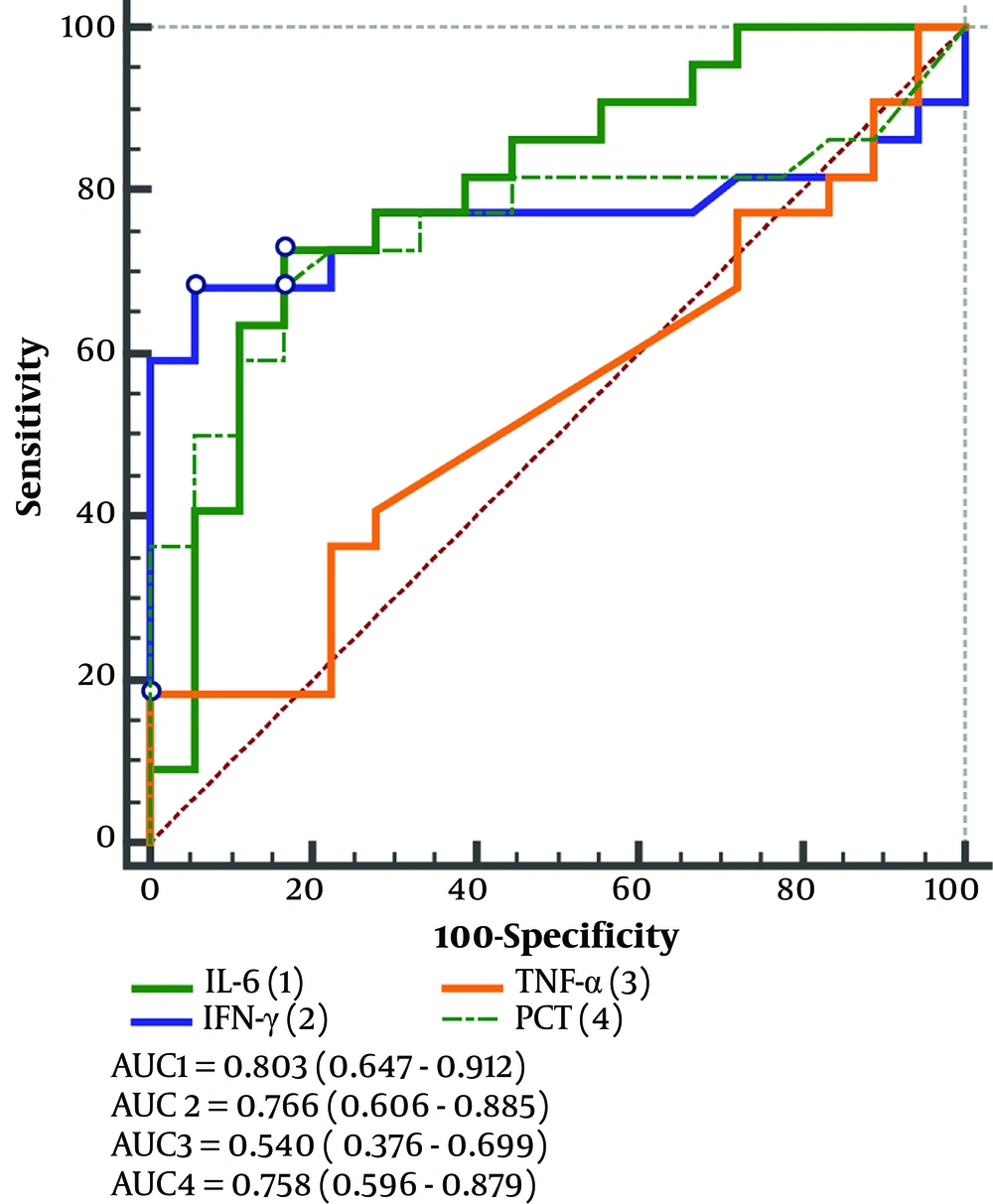
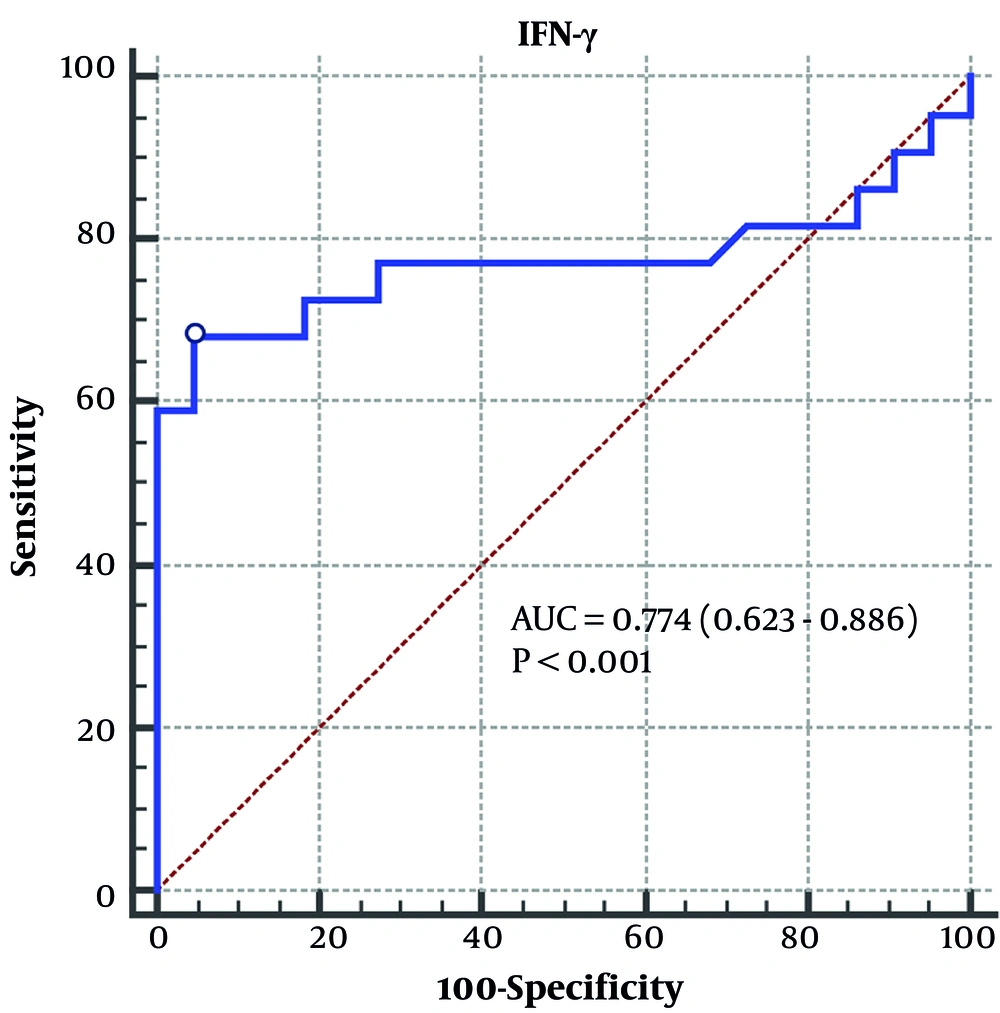
Further ROC curves were drawn for the inflammatory indicators mentioned in the above analysis. The AUC synthesizes the diagnostic efficacy of a diagnostic test at different thresholds. The ROC curve analysis showed that the AUC of inflammatory indicators IFN-γ, IL-6, and PCT were 0.766 (95% CI, 0.606 - 0.885), 0.803 (95% CI, 0.647 - 0.912), and 0.758 (95% CI, 0.596 - 0.879), respectively (Figure 2). The AUC of IFN-γ in predicting viral pneumonia was 0.774 (95% CI, 0.623 - 0.886) (Figure 3). These results suggest good diagnostic accuracy of IFN-γ in differentiating C. psittaci from viral pneumonia.
Sensitivity measures a diagnostic test's ability to identify disease, and specificity evaluates its effectiveness in distinguishing healthy individuals. The combination of the two is used to comprehensively evaluate the therapeutic efficacy and credibility of the indicator for the diagnostic test. The maximum Youden’s Index is calculated by sensitivity and specificity, and the corresponding cutoff value is obtained, which is used to distinguish normal and abnormal results. It can be seen that when the cutoff value was taken as IFN-γ > 16.26 pg/mL, the sensitivity and specificity of predicting the occurrence of C. psittaci pneumonia were 68.18% and 95.45%, respectively.
5. Discussion
The clinical presentations and laboratory findings of C. psittaci pneumonia and viral pneumonia closely resemble each other, posing challenges in their differential diagnosis (13, 14, 21). This study aimed to compare the clinical, laboratory, and radiological characteristics of patients diagnosed with C. psittaci pneumonia and viral pneumonia. Our analysis revealed that patients with C. psittaci pneumonia manifested a higher frequency of nervous system symptoms and elevated PCT levels. Conversely, individuals with viral pneumonia demonstrated higher lymphocyte percentages and a greater prevalence of bilateral lung lesions on chest CT scans. Moreover, we observed that the sensitivity, specificity, and AUC of IFN-γ in distinguishing C. psittaci pneumonia from viral pneumonia were determined to be 68.18%, 95.45%, and 0.766, respectively.
Given the striking resemblance in clinical symptoms, laboratory markers, and radiographic features between C. psittaci pneumonia and viral pneumonia, differentiation of these conditions can be challenging prior to microbiological confirmation (14, 21). Between 1890 and 1930, numerous severe human psittacosis outbreaks were found around the world, all associated with transient spillover infections in humans in close contact with parrots and other birds (22). Human-to-human transmission of C. psittaci was considered rare at the time. In two small outbreaks of C. psittaci (in Scotland in 2011 and in Sweden in 2013), only two confirmed secondary cases were identified (23, 24). The possibility of interpersonal transmission was added to the investigation by Zhang et al. Among the patients included in the study, the outbreak began with avian-to-human transmission, followed by secondary and tertiary human-to-human transmission, including by several asymptomatic carriers and health care workers (19). It is therefore important to recognize this as a major biosecurity issue and emerging infectious disease (5, 6, 19). Several nations have designated psittacosis as a notifiable infectious disease for surveillance and control purposes. Moreover, the treatment with antibiotics, prognoses, and supportive measures for C. psittaci differ significantly. Thus, early detection of distinct clinical features is imperative for distinguishing between the two diseases.
The clinical manifestations of C. psittaci pneumonia and viral pneumonia may be very similar, as both may present with symptoms such as fever, cough, and respiratory distress. There are limitations and non-specificity in the viral testing methods for both diseases. At the consultation stage, inadequate knowledge and experience regarding the disease might result in misdiagnosis or inappropriate treatment, leading to delayed or inappropriate treatment.
Our findings revealed both similarities and distinctions between C. psittaci and viral pneumonia. The overall distribution of these diseases based on demographic characteristics exhibited similarities, yet differences were observed in age and gender predisposition. Specifically, viral pneumonia predominantly affected older individuals, whereas C. psittaci pneumonia tended to afflict younger men. Additionally, we identified similar clinical presentations in both types of pneumonia, such as fever, cough, and sputum production. However, patients with C. psittaci pneumonia demonstrated a higher incidence of nervous system symptoms (45.5% vs 4.5%, P < .002), coupled with lower rates of respiratory virus infection symptoms (e.g., sputum) compared to those with viral pneumonia.
Our investigation also indicated elevated levels of inflammatory markers (neutrophil percentage, IFN-γ, and PCT) in patients with C. psittaci pneumonia compared to those with viral pneumonia. The Clinical Inflammatory Index was notably higher in C. psittaci pneumonia, suggesting a potentially more severe inflammatory response. Inhalation of C. psittaci triggers continuous replication in alveolar epithelial cells, leading to the release of pro-inflammatory chemokines. This process induces the recruitment and activation of macrophages, neutrophils, and other inflammatory cells, initiating an inflammatory cascade within the lungs (25).
In this retrospective study conducted at a single center, we observed a notable increase in serum IFN-γ levels among patients diagnosed with C. psittaci pneumonia compared to those with viral pneumonia. The diagnostic accuracy of IFN-γ suggests its potential as a valuable biomarker for differentiating between these two respiratory infections. Compared to IL-6 and PCT, IFN-γ can inhibit viral replication by activating antiviral mechanisms. Studies have shown that the level of IFN-γ may start to increase at the early stage of infection, which is important for early diagnosis. In contrast, the elevation of IL-6 and PCT lags relatively behind. These advantages make IFN-γ an important application in the diagnosis and therapeutic monitoring of distinguishing C. psittaci pneumonia from viral pneumonia. Such discoveries could aid in enhancing the prompt identification and treatment of both C. psittaci and viral pneumonia. The measurement of serum IFN-γ levels could serve as an additional tool in the diagnostic algorithm for these conditions.
The outcomes of this research align with prior evidence highlighting the significance of IFN-γ in mounting immune defenses against intracellular pathogens, such as C. psittaci and specific viral infections (26). The significantly higher levels of IFN-γ observed in patients with C. psittaci suggest a robust cellular immune response to the infection. Conversely, viral pneumonia is primarily caused by respiratory viruses that often trigger a different immune response, leading to lower IFN-γ levels compared to C. psittaci (27). The diagnostic performance of IFN-γ, assessed through ROC curve analysis, demonstrated promise with an AUC of 0.77. At the optimal cutoff value, IFN-γ exhibited a sensitivity of 68.18% and a specificity of 95.45%. These results suggest that measuring serum IFN-γ levels could assist clinicians in differentiating between C. psittaci and viral pneumonia, helping to guide appropriate treatment decisions and infection control measures.
With the development of NGS assays, it is possible to rapidly and accurately detect different pathogens in various samples, including atypical pathogens that may be difficult to culture in the laboratory (18). The NGS method can be used to obtain rapid etiologic results, make timely adjustments to tetracycline-based antimicrobial treatments, decrease the time to diagnosis of psittacosis, and shorten the duration of C. psittaci pneumonia (14, 28, 29). All patients with C. psittaci pneumonia in our study were diagnosed using NGS on blood and/or respiratory samples due to many limitations of commercial laboratory tests not included in routine clinical testing. However, NGS may still suffer from false positives, low detection rates of certain pathogens, and high costs, which particularly increase the economic burden on patients and limit its clinical use (29).
It remains critical to determine the similarities and differences in clinical presentation, laboratory findings, and imaging features between C. psittaci and symptomatically similar pneumonia diseases. It is crucial to acknowledge the limitations of this study. Firstly, its retrospective nature introduces inherent biases and constraints associated with this type of investigation. Prospective studies are necessary to corroborate the diagnostic efficacy of IFN-γ and to explore its applicability in clinical settings. Secondly, the study was limited to one center and included only 22 cases of C. psittaci, which may limit the generalizability of the findings. This small sample size did not allow exploration of all relevant features of C. psittaci, limiting the identification of more subtle differences between patients with C. psittaci pneumonia and viral pneumonia. We did not perform a risk factor analysis in the current study because of the many confounding factors in retrospective studies.
Although it has been concluded that IFN-γ is a possible biomarker for differentiating C. psittaci pneumonia from viral pneumonia, its benefits as a clinical management strategy need to be validated in prospective studies with large sample sizes. Multicenter studies involving larger patient populations are necessary to validate these results in different populations. Moreover, the study only included cross-sectional samples from cases of C. psittaci pneumonia and viral pneumonia, without collecting longitudinal samples. Therefore, the results obtained could not resolve the kinetics of the markers. Finally, clinical follow-up data were not obtained from patients after discharge from the hospital, and future studies aim to evaluate the long-term effects or outcomes of C. psittaci infection.
5.1. Conclusions
This single-center retrospective study provides evidence supporting the role of serum IFN-γ levels as a potential biomarker for differentiating C. psittaci from viral pneumonia. The notable increase in IFN-γ levels among patients with C. psittaci indicates its role in the immune defense against this intracellular pathogen. Additional research is needed to validate these observations and ascertain the practical application of IFN-γ in the prompt identification and treatment of both C. psittaci and viral pneumonia. Incorporating enzyme-linked immunosorbent assay measurements of IFN-γ > 16.26 pg/mL in the blood into the diagnostic algorithm can help accurately identify the underlying cause of respiratory tract infections in a timely manner, thereby improving the prognosis of patients.