1. Background
Severe acute respiratory syndrome (SARS)-CoV-2 infection causes the novel coronavirus, known as COVID-19, which shares close ties with SARS-CoV. It is the third coronavirus-caused zoonotic disease affecting humans, following SARS and Middle East respiratory syndrome (MERS) (1, 2). The initial cases of infection with this virus were documented at a seafood market in south Wuhan in December 2019 (3), and it was ultimately unfeasible to exclude the possibility of human-to-human transmission. Cytokines are polypeptide signaling molecules that regulate various biological processes through cell surface receptors (4). In response to stress-inducing internal processes, such as microbial infections, host cells produce cytokines, which are crucial for reprogramming cell metabolism as a protective response (5-7).
Regarding COVID-19, the immune response is believed to involve a complex interplay between pro-inflammatory and anti-inflammatory cytokines. Several studies have reported abnormal levels of cytokines and chemokines in patients affected by COVID-19 (8). Early in the pandemic, Blanco-Mello et al. revealed that an inappropriate and weak immune response appeared more frequently in patients with comorbidities (9). There may be a turning point in SARS-CoV-2 infection when the body's natural defenses against viruses are weakened and inflammatory cytokines are produced in large amounts (9). In many patients with severe COVID-19, higher serum levels of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-2, and IL-6, as well as chemokines like IL-8, have been observed compared to those with mild disease (10, 11). This suggests that the pathogenesis of COVID-19 involves an imbalance in T-helper cell (TH) subsets, including TH1, TH2, and TH17, as well as regulatory T-cells (Tregs).
2. Objectives
Owing to the limited data available on cytokine profiles in Sudanese COVID-19 patients, this study was conducted to evaluate the serum levels of TNF-α, IL-4, IL-6, IL-10, and IL-17 in Sudanese COVID-19 patients compared to a healthy control group in Kassala State, Eastern Sudan.
3. Methods
3.1. Study Design and Specimen Collection
A case-control study was conducted in Kassala State, Eastern Sudan, between September 2020 and January 2021. Individuals suspected of having COVID-19 who visited the COVID-19 isolation center at Kassala Teaching Hospital (KTH) provided informed verbal consent during registration. All patients (n = 90, male = 68 and female = 22) admitted to the hospital, clinically suspected and confirmed positive for SARS-CoV-2 via RT-PCR, were included in the study, irrespective of age. Disease severity was assessed upon admission and categorized as mild (n = 65), moderate (n = 16), or severe (n = 9) COVID-19, following the criteria set by the World Health Organization (12). Mild disease is characterized by the absence of evidence of viral pneumonia or hypoxia. Moderate disease involves clinical signs of pneumonia, such as fever, cough, dyspnea, and rapid breathing, without severe pneumonia, specifically maintaining SpO2 ≥ 90% on room air. Severe disease is defined by clinical signs of pneumonia alongside one or more of the following: Respiratory rate exceeding 30 breaths/minute, severe respiratory distress, or SpO2 < 90% on room air.
Patients who had received a vaccination against SARS-CoV-2 or were undergoing treatment with antibiotics, steroids, immunosuppressives, or other therapeutics that could influence inflammatory mediators and outcomes were excluded from the study. The controls (n = 90, male = 57 and female = 33) were selected from hospital staff who had negative RT-PCR results and had not been in contact with individuals suspected of having COVID-19 for at least two weeks prior to sampling. Data collection utilized a pre-designed questionnaire that included questions about age, occupation, residence, educational attainment, symptoms and signs presented by patients, and their medical history. Blood samples were collected in plain containers, and serum was obtained by allowing blood to coagulate for 30 minutes before centrifugation at 3000 rpm for 10 minutes. The aliquots were stored at -20°C until testing began.
3.2. Cytokines Assay
The enzyme-linked immunosorbent assay (ELISA) kits for human IL-4, IL-17, IL-10, IL-6, and TNF-α were obtained from Sunlong Biotech Co., Ltd. (Zhejiang, China). The sensitivity of the IL-4 and IL-17 kits is 0.8 pg/mL, while the sensitivity of the IL-10 kit is 0.3 pg/mL, the IL-6 kit is 0.5 ng/mL, and the TNF-α kit is 2.2 pg/mL. The analysis was performed following the manufacturer’s instructions for each ELISA kit. The optical densities (OD) were measured using a microplate ELISA reader (Stat Fax 4200, USA) at a wavelength of 450 nm.
3.3. Statistical Analysis
The data were analyzed using the statistical package for the social sciences (SPSS) software (IBM version 20.0, USA). Categorical data were presented as numerical counts, frequencies, and proportions. Means and standard errors of the means (SEM) were calculated for all continuous variables. Comparisons between patient groups were conducted using one-way ANOVA followed by Tukey's post-hoc analysis. Receiver operating characteristic (ROC) curve analysis was employed to determine the optimal cut-off values of cytokine levels, offering the highest sensitivity and specificity in detecting the severity of COVID-19. Graphs were generated using GraphPad Prism 8 software along with SPSS. The threshold for statistical significance was set at a P-value of less than 0.05.
4. Results
The demographic data and clinical characteristics of the study subjects are presented in Table 1. Male participants outnumbered female participants in the study groups, comprising 69.4% of the total. The majority of the study subjects were aged between 20 and 29 years (43.9%), resided in metropolitan areas (91.1%), and had a high level of education (67.2%). Table 2 indicates that 72.2% of the patients exhibited mild illness. A significant percentage of patients presented symptoms such as fever (84.4%), sore throat (58.9%), cough (54.4%), and headache (51.1%), with a higher prevalence observed in both severe and mild cases. Diabetes mellitus (37.5%) and chronic lung illness (35.3%) were identified as common comorbidities in both severe and mild cases.
| Characteristics | Cases (n = 90) | Controls (n = 90) |
|---|---|---|
| Gender | ||
| Male | 68 (75.6) | 57 (63.3) |
| Female | 22 (24.4) | 33 (36.7) |
| Age (y) | ||
| < 10 | 1 (1.1) | 1 (1.1) |
| 10 - 19 | 2 (2.2) | 2 (2.2) |
| 20 - 29 | 38 (42.2) | 41 (45.6) |
| 30 - 39 | 12 (13.3) | 15 (16.7) |
| 40 - 49 | 10 (11.1) | 6 (6.7) |
| 50 - 59 | 10 (11.1) | 6 (6.7) |
| 60 - 69 | 7 (7.8) | 11 (12.2) |
| > 70 | 10 (11.1) | 8 (8.9) |
| Residence | ||
| Rural | 7 (7.8) | 9 (10.0) |
| Urban | 83 (92.2) | 81 (90.0) |
| Education | ||
| Illiterate | 6 (3.3) | 9 (5.0) |
| Primary | 8 (4.4) | 10 (5.6) |
| Secondary | 14 (7.8) | 12 (6.7) |
| University | 62 (34.4) | 59 (32.8) |
| Patient status | ||
| Recovered | 76 (84.4) | - |
| Deceased | 14 (15.6) | - |
a Values are expressed as No. (%).
| Characteristics | Total (n = 90) | Severe (n = 9) | Moderate (n = 16) | Mild (n = 65) |
|---|---|---|---|---|
| Gender | ||||
| Male | 68 (75.6) | 6 (66.7) | 10 (62.5) | 52 (80) |
| Female | 22 (24.4) | 3 (33.3) | 6 (37.5) | 13 (20) |
| Residence | ||||
| Urban | 81 (90.0) | 7 (77.8) | 15 (93.8) | 61 (93.8) |
| Rural | 9 (10.0) | 2 (22.2) | 1 (6.2) | 4 (6.2) |
| Education | ||||
| Illiterate | 5 (55.6) | 2 (22.2) | 2 (12.6) | 1 (1.6) |
| Primary | 8 (8.9) | 1 (11.1) | 1 (6.2) | 6 (9.2) |
| Secondary | 14 (15.6) | 1 (11.1) | 6 (37.6) | 7 (10.8) |
| University | 62 (68.9) | 5 (55.6) | 7 (43.8) | 50 (77.0) |
| Symptoms at admission | ||||
| Fever | 76 (84.4) | 9 (100) | 16 (100) | 51 (78.5) |
| Cough | 49 (54.4) | 7 (77.8) | 13 (81.3) | 29 (44.6) |
| Dyspnoea | 22 (24.4) | 9 (100) | 13 (81.3) | 0 (0) |
| Sore throat | 53 (58.9) | 4 (44.4) | 13 (81.3) | 36 (55.4) |
| Headache | 46 (51.1) | 4 (44.4) | 11 (68.8) | 31 (47.7) |
| Myalgia | 36 (40) | 4 (44.4) | 3 (18.8) | 29 (44.6) |
| Runny nose | 10 (11.1) | 1 (11.1) | 3 (18.8) | 6 (9.2) |
| Comorbidity | ||||
| Diabetes mellitus | 40 (44.4) | 7 (77.8) | 8 (50) | 25 (38.5) |
| Hypertension | 4 (4.4) | 2 (22.2) | 2 (12.5) | 0 (0) |
| Renal impairment | 3 (3.3) | 0 (0) | 1 (6.3) | 2 (3.1) |
| Chronic pulmonary disease | 17 (18.9) | 3 (33.3) | 3 (18.8) | 11 (16.9) |
| Neurological disorder | 9 (10.0) | 0 (0) | 2 (12.5) | 7 (10.8) |
| Asthma | 7 (7.8) | 1 (6.3) | 1 (6.3) | 5 (7.7) |
| Epilepsy | 9 (10.0) | 7 (10.8) | 2 (12.5) | 0 |
| Tuberculosis | 10 (11.1) | 2 (22.2) | 2 (12.5) | 6 (9.2) |
a Values are expressed as No. (%).
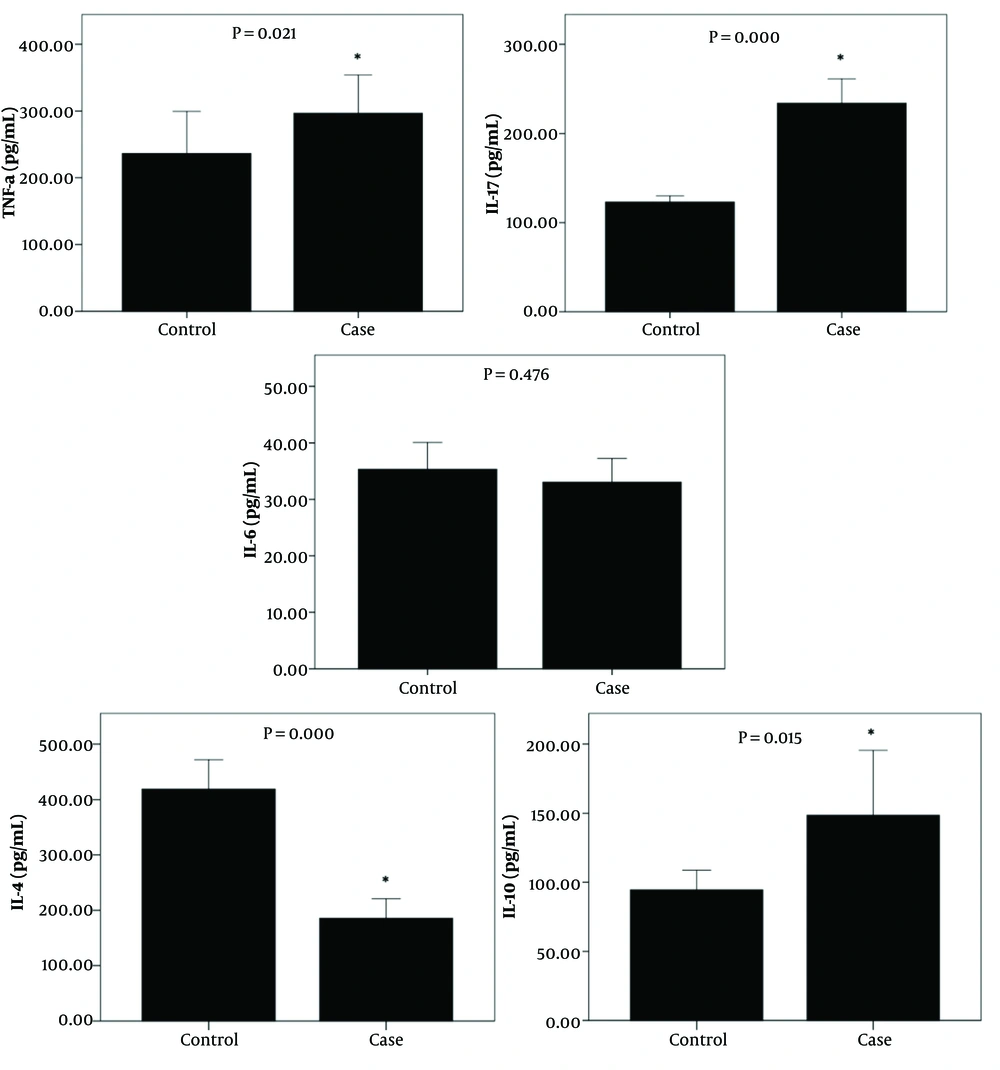
Patients showed significantly higher levels of TNF-α, IL-17, and IL-10 (301.20 ± 29.76 vs. 236.32 ± 31.55, P = 0.021; 224.70 ± 04.52 vs. 123.05 ± 03.46, P = 0.000; and 144.86 ± 13.26 vs. 89.58 ± 7.55, P = 0.015, respectively) compared to the control group. Conversely, IL-4 levels were significantly higher in the control group than in the patient group (418.91 ± 26.43 vs. 188.01 ± 18.75, P = 0.000) (Figure 1).
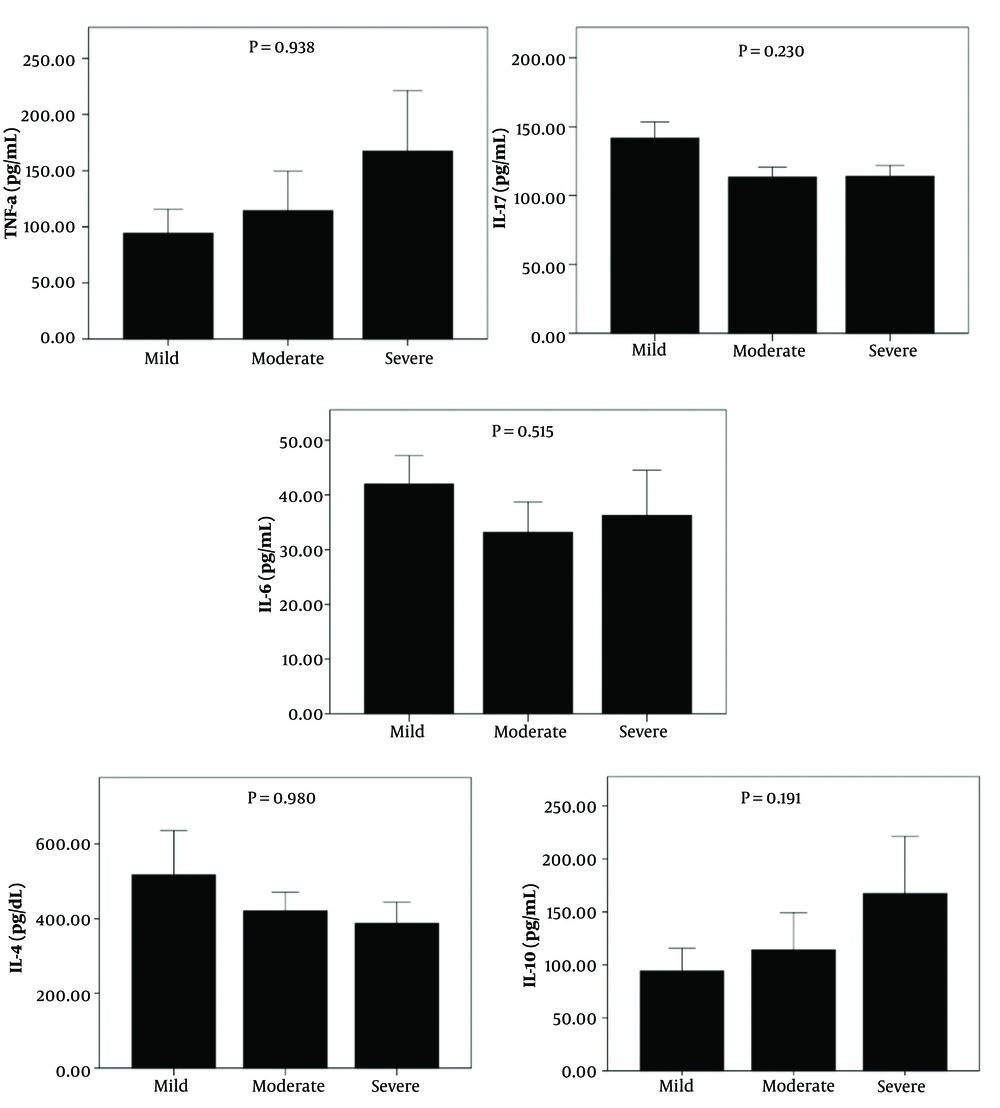
Analysis of the mild, moderate, and severe groups revealed that TNF-α and IL-10 levels were elevated in the severe group compared to the mild and moderate groups. The levels of IL-4 were higher in the mild group compared to the moderate and severe groups. However, these differences did not reach statistical significance. No changes were observed in IL-17 and IL-6 levels among the study groups (Figure 2).
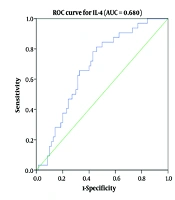
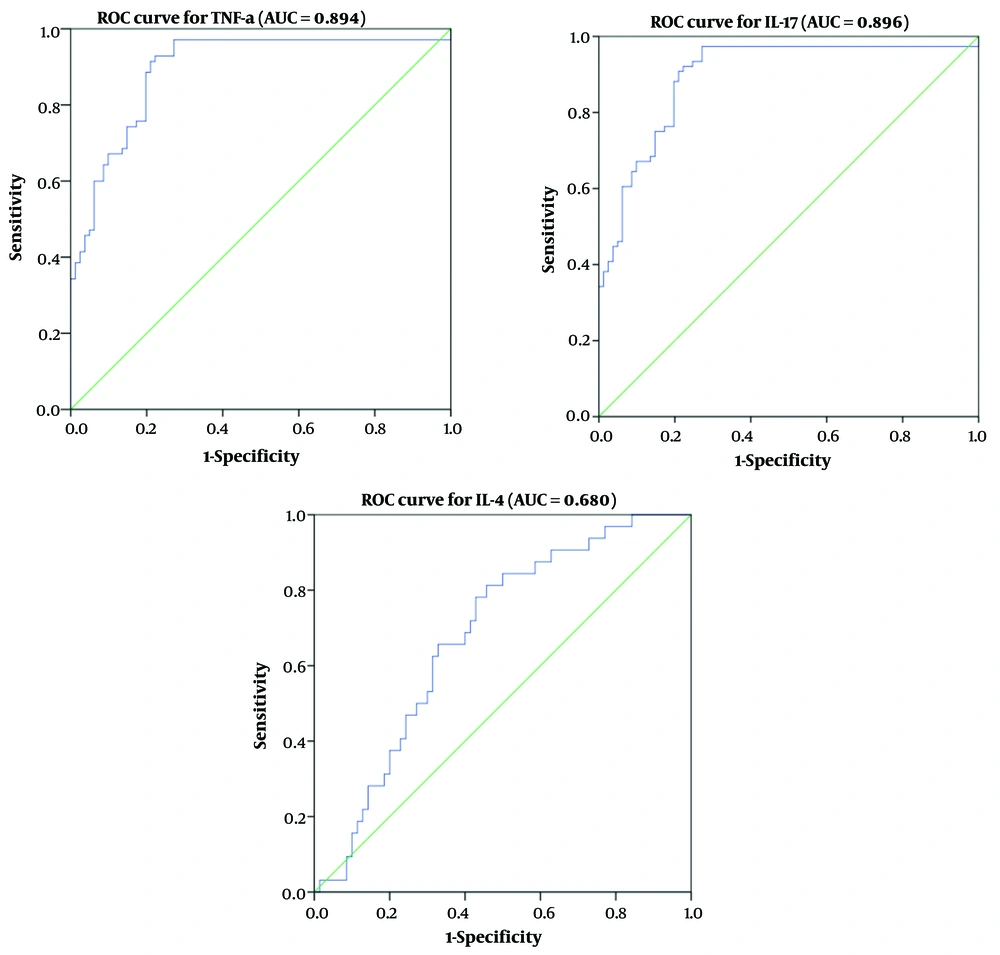
The diagnostic utility of the inflammatory mediators TNF-α, IL-17, and IL-4 was evaluated using ROC curve analysis, including the area under the ROC curve (AUC), to predict the severity of COVID-19 (Table 3 and Figure 3). The AUC values for IL-17, TNF-α, and IL-4 were 0.894, 0.896, and 0.680, respectively. Receiver operating characteristic analysis indicated a correlation between serum TNF-α (> 417.4), IL-17 (> 396.7), and IL-4 (> 232.1) levels and disease severity. Tumor necrosis factor-α exhibited the highest sensitivity, while IL-17 demonstrated the greatest specificity in predicting disease severity.
| Variables | AUC (95% CI) | Cut-off | Sensitivity | Specificity | P-Value |
|---|---|---|---|---|---|
| TNF-a | 0.894 (0.767 - 0.906) | > 417.4 | 88.2 | 91.6 | < 0.001 |
| IL-17 | 0.896 (0.815 - 0.921) | > 396.7 | 87.0 | 94.3 | < 0.001 |
| IL-4 | 0.680 (0.609 - 0.761) | > 232.1 | 55.1 | 82.1 | < 0.004 |
Abbreviations: AUC, area under the curve; TNF, tumor necrosis factor; IL, interleukin.
5. Discussion
Researchers globally face challenges in comprehensively understanding various aspects of COVID-19, and the knowledge regarding its immunopathological mechanisms is continually developing. Cytokines and their receptors play a significant role in the pathology of viral infections (13). A prior study indicated a correlation between the severity of COVID-19 and cytokine storms (14). In this study, we aimed to assess the serum levels of specific cytokines in Sudanese patients diagnosed with COVID-19.
The findings revealed that COVID-19 patients exhibited significantly elevated TNF-α levels compared to the control group, suggesting a pro-inflammatory response. This aligns with studies reporting increased serum levels of TNF-α in COVID-19 patients compared to controls (15-18). Additional research has identified a correlation between elevated plasma levels of TNF-α and IL-6 and post-acute sequelae of COVID-19 (19). The production of elevated TNF-α signifies a Th1 cell response in patients infected with SARS-CoV-2, suggesting that a robust response may contribute to better outcomes for COVID-19 patients.
This study also found significantly elevated levels of IL-17 in COVID-19 patients compared to controls, consistent with findings by Guo et al., which indicated that pro-inflammatory cytokines, including IL-1β, IL-6, IP-10, G-CSF, IL-8, IL-17, and IFN-γ, were markedly increased in COVID-19 patients (20). Individuals experiencing long COVID-19 exhibit significantly elevated levels of IL-17 and IL-2, while showing reduced levels of IL-4 and IL-10 (21). However, other studies have reported negligible differences in IL-17 levels between COVID-19 patients and controls (22). Interleukin -17 is crucial in modulating neutrophil responses by inducing IL-8 release, which acts as a potent chemoattractant for neutrophils in the context of acute lung injury (23). A report by Gouda et al. indicates a significant correlation between acute lung injury in patients with severe COVID-19 and T-helper type 17 (Th17) cell responses, as IL-17 may impair normal alveolar function by inducing apoptosis (24).
The present study demonstrates that IL-4 levels were markedly reduced in COVID-19 patients compared to the control group. Data concerning IL-4 levels in COVID-19 patients remains contentious. Queiroz et al. observed that serum IL-4 levels were significantly reduced in COVID-19 patients relative to the post-COVID-19 cohort (21). Similarly, Williams et al. reported significantly lower IL-4 levels in COVID-19 patients compared to controls (25). Conversely, previous studies indicate significant expression of IL-4 in COVID-19 patients relative to the control group (16, 26). Other studies found no significant difference in IL-4 levels between COVID-19 cases and controls (27, 28). A reduced level of IL-4 in COVID-19 cases may suggest a shift in the immune response toward a Th1 profile, which is linked to further health complications in individuals who have undergone acute COVID-19 and may act as a molecular marker for long COVID-19 (16).
The present study indicates that COVID-19 patients exhibit significantly higher levels of IL-10 compared to controls, aligning with multiple studies that reported increased levels of IL-10 in COVID-19 patients relative to healthy individuals. This supports the hypothesis that IL-10 may be implicated in the pathogenesis of COVID-19 (16-18, 26, 27). In contrast, prior research has noted lower IL-10 levels in COVID-19 patients relative to individuals experiencing long COVID sequelae (16). IL-10, an anti-inflammatory cytokine, exhibits a significant increase during the cytokine storm, representing a secondary, yet counter-regulatory, response to pro-inflammatory cytokines (29).
Our findings indicate a rise in TNF-α, IL-17, and IL-10 and a reduction in IL-4 levels with disease severity compared to mild and moderate conditions, although the differences are not statistically significant. Conflicting reports have been stated regarding this matter; for instance, similar results showed that patients with severe COVID-19 exhibited no significant differences in the levels of TNF-α, IL-17, IL-10, and IL-4 in comparison to those with mild disease (21, 28, 30). Other studies revealed a significant correlation of TNF-α (21, 31), IL-17 (21), IL-6 and IL-10 (16), IL-4 and IL-10 (26), IL-10 (17, 18, 32), or IL-4 (27) levels with the severity of COVID-19.
This study represents the inaugural evaluation of serum levels of specific cytokines in Sudanese patients diagnosed with COVID-19. The elevated levels of TNF-α, IL-17, and IL-10 and reduced levels of IL-4 appear to constitute a cytokine profile associated with long-term COVID-19. Identifying these markers as potential targets may enhance treatment and prevention strategies for the disease in our population.
The current study has limitations, including a small sample size, the high cost of cytokine assays, and the wide disparity between Sudanese ethnic groups, all of which could influence the results. Further investigation into the role of cytokines in COVID-19 is essential, requiring a larger sample size from diverse regions in Sudan and including various cytokine profiles throughout disease progression.
5.1. Conclusions
The notable elevations in TNF-α, IL-17, and IL-10 levels, alongside the significant reduction in IL-4, may serve as indicators of adverse outcomes in COVID-19 patients. TNF-α, IL-17, and IL-4 were identified as potential predictors of disease severity in COVID-19 patients and may serve as therapeutic targets for managing the condition. Our findings suggest that cytokine measurement can act as an important indicator of disease progression and may contribute to developing more definitive and targeted treatment approaches.