1. Introduction
Acquired immune deficiency syndrome, when combined with opportunistic infections, carries a high risk of poor prognosis and high mortality, especially among patients with central nervous system infections (1). Toxoplasma gondii, an obligate intracellular parasite, is known to latently infect one-third of the world’s population. Acute T. gondii infections are usually subclinical in individuals with normal immune system function, with severe clinical symptoms rarely observed in such cases. However, in immunocompromised hosts, particularly those with acquired immune deficiency syndrome, it can lead to severe conditions. Computed tomography (CT) and MRI findings in patients with cerebral toxoplasmosis often reveal single or multiple ring-enhancing lesions in the brain with peripheral edema. Toxoplasma gondii, a common brain-tropic parasite in vivo, is primarily found in neurons (2). In rare instances, T. gondii infection can cause ventriculitis and necrosis, leading to the obstruction of pathways for cerebrospinal fluid circulation, such as the Sylvius aqueduct and foramen of Monro, resulting in obstructive hydrocephalus (3).
2. Case Presentation
A 34-year-old male with no prior medical history was admitted to a local hospital with progressive nausea and vomiting persisting for 9 days, along with headaches, dizziness, and blurred vision for 2 days. A CT examination revealed enlargement of the lateral ventricles and hypodensity in the right frontal lobe and the genu of the corpus callosum. A human immunodeficiency virus (HIV) test returned positive, prompting his transfer to our hospital. Upon admission, the patient exhibited decreased consciousness and positive meningeal irritation. The ophthalmic examination revealed only papilledema. Lung tests showed no abnormalities. Blood tests showed neutrophils 6.26 × 109/L, lymphocytes 0.28 × 109/L, and CD4+ T cells 5/μL. A cranial CT scan depicted bilateral enlargement of the lateral ventricles, with the left ventricle being more prominently enlarged, along with irregular ventricular walls.
When the patient was admitted to our hospital, an intracranial infectious lesion was suspected, prompting an immediate lumbar puncture after IV mannitol administration for dehydration and intracranial pressure reduction. The intracranial pressure measured 240 mmH2O, and colorless, clear cerebrospinal fluid was obtained and sent for examination. The analysis of cerebrospinal fluid revealed a nucleated cell count of 26 × 106/L and a protein level of 271.6 mg/dL. Additionally, the results of the Gram stain, India ink staining, cryptococcal antigen, and bacterial, mycobacterial, and fungal smears of cerebrospinal fluid were all negative. Both blood and cerebrospinal fluid tests for syphilis produced negative results. The cerebrospinal fluid was qualitatively positive for Toxoplasma-specific antibodies. Cerebral toxoplasmosis was suspected based on cerebrospinal fluid findings. Sulfamethoxazole (SMZ), trimethoprim (TMP), and clindamycin were administered for treatment, along with steroid hormones and mannitol to reduce cranial pressure. After two days, the IgG antibody to T. gondii in the cerebrospinal fluid exceeded 400 IU/mL, and targeted next-generation sequencing (tNGS) (Hangzhou Adicom Medical Laboratory Center) detected 246,262 T. gondii sequences in the cerebrospinal fluid (relative abundance 67.49%). The patient was diagnosed with T. gondii infection.
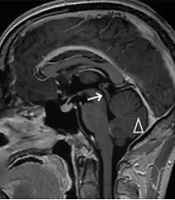
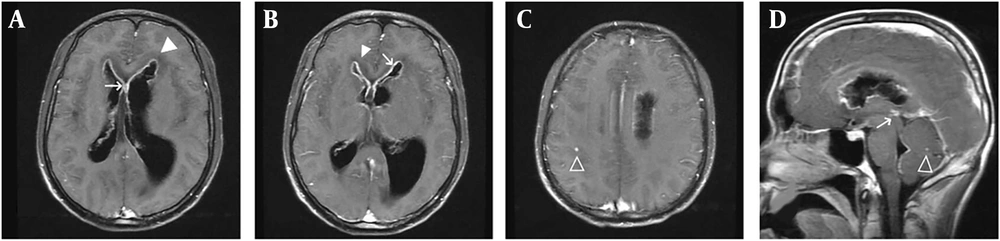
Enhanced magnetic resonance imaging revealed significant enhancement of the cerebral ventricular wall, peripheral interstitial cerebral edema, and small focal enhancing lesions in the brain parenchyma. Ventriculomegaly was pronounced, particularly in the left ventricle, suggesting possible blockage of the proximal opening of the midbrain aqueduct (Figure 1). Three days after continuing the above treatment, the patient’s level of consciousness deteriorated further. A repeat CT examination showed progressive aggravation of ventricular system dilation compared with that observed 5 days earlier. After obtaining informed consent from the patient’s relatives, preoperative ventriculoperitoneal shunting (VPS) was performed. On the first postoperative day, the patient regained consciousness.
The headache was significantly reduced, and there was good movement of the limbs. Three days after surgery, the diagnosis of human acquired immunodeficiency disease was reconfirmed by the Centers for Disease Control and Prevention, and highly active antiretroviral therapy (HAART) was initiated with tenofovir, lamivudine, and dolutegravir. On follow-up CT one week after surgery, significant improvement in the thickening of the ventricular wall was observed, and the dilation of the ventricular system had resolved. The patient was discharged in good condition.
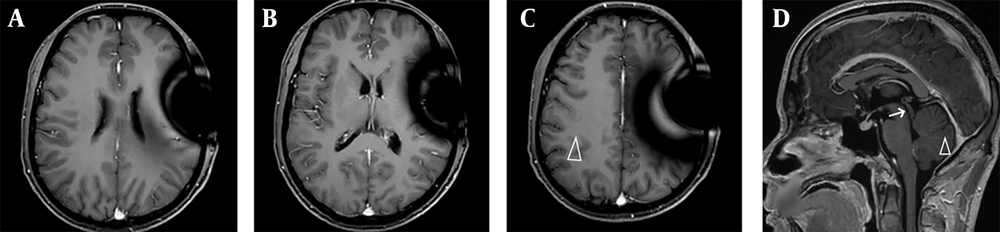
After discharge, SMZ, TMP, and azithromycin were administered orally. Enhanced MRI six weeks after surgery showed that ventricular dilation had resolved, enhancement of the ventricular wall had completely subsided, and the original intracerebral enhancing nodules had disappeared (Figure 2). The patient was clinically cured.
3. Discussion
Toxoplasma gondii is a brain-infecting parasite capable of infecting most nucleated cells in vitro, including astrocytes and neurons. However, T. gondii is primarily found in neurons in vivo. A study from the University of Arizona suggested that neurons are not randomly infected but are the main target cells for T. gondii in the body (2). Toxoplasmosis is a prevalent opportunistic infection of the central nervous system (CNS) in HIV-positive patients. Even in the HAART era, CNS toxoplasmosis remains highly prevalent, particularly in cases of severe immunosuppression, and it represents a leading cause of morbidity and mortality. The most common symptoms include single or multiple ring-enhancing lesions, typically located at the junction of gray matter and white matter, within white matter or the basal ganglia, or presenting as diffuse encephalitis (4). The main neuropathologic feature observed in people living with HIV (PLWHA) and cerebral toxoplasmosis is multifocal necrotizing encephalitis (5).
The most recognized association between cerebral toxoplasmosis and HIV lies in the disturbance of the antiparasitic T-cell response to parasites in immunocompromised patients, resulting in an inability to control the persistent intracellular parasite. Cerebral toxoplasmosis combined with hydrocephalus is more commonly observed in children infected with congenital toxoplasmosis (3, 6, 7), whereas it is rare for HIV patients to have cerebral toxoplasmosis and hydrocephalus. The possible mechanisms of hydrocephalus involve peripheral substantial space-occupying lesions, necrotizing ventriculitis, and choriomeningitis, leading to blockage of the foramen of Monro. This obstruction is manifested by unilateral or bilateral ventriculomegaly (8), particularly around the aqueduct of Sylvius. Even mild inflammation can obstruct the aqueduct of Sylvius, blocking the flow of cerebrospinal fluid. Both causes might coexist (3, 9).
The diagnosis of cerebral toxoplasmosis relies on typical imaging findings, and serologic and cerebrospinal fluid-specific antibody tests are often employed to confirm the diagnosis. Molecular diagnostics are increasingly applied in clinical practice. The specificity of T. gondii DNA in the cerebrospinal fluid of patients with PLWHA ranges from 96% to 100%, with a positive predictive value of 100% and a negative predictive value of 71% to 92% (10, 11). Despite its moderate sensitivity in most studies, the high specificity and precise positive predictive value of the PCR assay render it a valuable tool for diagnosing cerebral toxoplasmosis. In our case, we expedited the diagnosis of T. gondii infection using targeted next-generation sequencing technology. Targeted NGS is a high-throughput sequencing technology targeting a specific gene or genome. The tNGS focuses on specific gene sequences for high-throughput sequencing to improve detection sensitivity and eliminate host nucleic acid interference. It has the characteristics of high throughput, fast detection, and low cost. A study from the United States discovered that the T. gondii matrix antigen (MAG1) could predict toxoplasmic encephalitis (TE) in HIV-infected patients (12).
In this case, SMZ and TMP were prescribed as the primary anti-infective medication regimen for the patient, supplemented with clindamycin for anti-infective therapy. Several studies have not definitively established the comparative efficacy and safety between the pyrimethamine combined with sulfadiazine (P-S) regimen and the pyrimethamine combined with clindamycin (P-C) regimen (13). However, the patient’s condition deteriorated after treatment with these drugs, and imaging findings revealed progressive ventricular enlargement. As a result, VPS was performed. Postoperatively, the patient regained consciousness, and imaging manifestations improved rapidly. Sulfamethoxazole and TMP in combination with azithromycin were administered to the patient for another 6 weeks for anti-infective therapy. Subsequently, clindamycin was replaced with azithromycin due to the inability to administer clindamycin via an enteral tube.
There are few reports on cases of HIV-related cerebral toxoplasmosis with hydrocephalus. Some scholars believe that early diagnosis and initiation of anti-infective treatment might obviate the need for surgical interventions such as shunting procedures (14). However, most researchers contend that early surgical intervention for patients with cerebral toxoplasmosis combined with ventriculitis and hydrocephalus is crucial for an excellent prognosis (3, 15, 16). Despite debates regarding the optimal surgical approach, it has been suggested that the high protein concentration in the cerebrospinal fluid might impact the success of VPS. Therefore, performing ventricular lavage before the shunting procedure may enhance the success rate of VPS (16). Some studies have also suggested that endoscopic third ventriculostomy (ETV) is more effective (3, 15, 16). However, it may lead to the spread of inflammation to the basal cistern and spinal cord subarachnoid space, potentially blocking the flow of cerebrospinal fluid in the spinal cord subarachnoid space (16).
3.1. Conclusions
This case suggests that early surgical intervention may be beneficial for the prognosis of patients with HIV-related toxoplasmic encephalopathy and hydrocephalus when they fail to respond to standardized anti-infective therapy.