1. Background
Toxoplasmosis is a common parasitic disease caused by the obligate intracellular parasite Toxoplasma gondii. The definitive hosts of this protozoan are cats and other felines, while warm-blooded animals (birds and mammals) serve as intermediate hosts (1). This zoonotic disease has a global distribution and is typically asymptomatic and self-limiting in individuals with normal immunity. However, in immunocompromised hosts, the disease prognosis is poor, with 23% developing Toxoplasma encephalitis (2). Additionally, if a mother is infected with toxoplasmosis during pregnancy, the disease can lead to complications such as abortion, hydrocephaly or microcephaly, blindness, jaundice, and chorioretinitis (3).
Despite the high importance of managing and treating toxoplasmosis in medicine, few drugs are effective (4). The antimicrobial treatment of choice for toxoplasmosis is sulfadiazine and pyrimethamine. However, these drugs have teratogenic effects on the fetus, can suppress the bone marrow system, and may cause blood poisoning and kidney problems. Furthermore, they are ineffective in eliminating tissue cysts (5). Therefore, the development of an effective vaccine is crucial from both medical and veterinary perspectives.
An effective human vaccine should not only reduce infection and mortality rates but also lessen the burden of chronic cases requiring long-term care, while being economically viable (6). It is known that immunity to Toxoplasma increases after infection, with both humoral and cellular immunity playing important roles in immunogenesis (7). Over the last 30 years, significant efforts have been made to develop vaccines against toxoplasmosis using various strategies, including killed and attenuated organisms, tachyzoite lysate antigen, excretory-secretory (ES) antigens, somatic proteins, DNA vaccines, and recombinant proteins. However, none of these methods have produced acceptable immunity among antigens tested as vaccines (6, 8-10).
Despite extensive efforts in vaccine development, only the commercial vaccine TOXOVAX has been released to the market (6). This subunit vaccine contains live attenuated tachyzoites from the RH strain, known as 48S, which have lost their ability to form tissue cysts in the host (11). TOXOVAX is used only in veterinary medicine, with disadvantages including its lack of use in humans, especially during pregnancy, and its short-term effect. Vaccination with the RH strain in mice has been unsuccessful, although some other strains have shown limited success when combined with immune stimulation (12).
The use of ionizing radiation, including gamma rays, has long been considered in the development of drugs and vaccines. This technology is used in the development of human and animal vaccines, sterilization, and the production of random mutations (13, 14). It has been shown that gamma rays can destroy the infectivity of T. gondii cysts and that other electromagnetic rays, such as ultraviolet rays, have an inhibitory effect on Toxoplasma the tachyzoites, affecting cell proliferation and tissue cyst formation (15, 16). By exposing Toxoplasma the tachyzoites to controlled doses of gamma radiation, their ability to cause disease may be weakened while maintaining their antigenic properties. Thus, gamma radiation-attenuated tachyzoites could serve as a vaccine candidate.
2. Objectives
Despite significant progress in developing vaccines against infectious agents, effective vaccines for protozoa, especially in humans, have not been introduced. Therefore, considering the importance of toxoplasmosis, this study aims to use gamma radiation-attenuated tachyzoites of the T. gondii RH strain as an effective vaccine.
3. Methods
3.1. Tachyzoites Preparation
Intraperitoneal passages in female BALB/c mice were used for the preparation of the RH strain of T. gondii (17). Briefly, 2 × 105 tachyzoites of the RH strain were injected intraperitoneally into 5 mice. After 4 - 5 days, peritoneal fluid was aspirated from the infected mice in phosphate-buffered saline (PBS) at pH 7.2. The tachyzoites were then washed and purified by centrifugation.
3.2. HeLa Cell Line Culture
The HeLa cell line, stored in liquid nitrogen in the Parasitology Department of Shiraz University of Medical Sciences, was removed from the tank and immediately recovered. DMEM medium (Sigma, USA) enriched with 10% heat-inactivated fetal calf serum (FCS; Gibco, USA) and 100 IU/mL penicillin-100 μg/mL streptomycin (Roche) was used to cultivate the cells in 25 cm2 flasks (Corning Costar, UK). The cells were maintained in 5% CO2 at 37°C and 80% humidity and were subcultured by trypsinization and washing with PBS (pH 7.2) once every three days (18).
3.3. Irradiation of Toxoplasma gondii Tachyzoites
At the Shiraz Blood Transfusion Organization in Shiraz, Iran, the purified tachyzoites suspended in PBS from the previous stage were irradiated with gamma rays at a rate of 1.5 Gy/min from a distance of 40 cm. The radiation doses were 50, 100, and 200 Gy (19).
3.4. Evaluating the Effect of Different Dose of Gamma Ray on Tachyzoites
The rate of apoptosis in the irradiated tachyzoites was estimated using the flow cytometry method (20). Briefly, 2 × 105 irradiated tachyzoites of the T. gondii RH strain were counted from each dose of gamma rays. Propidium iodide (Sigma, USA) was then added at a final concentration of 50 μg/mL to each tube and incubated in the dark at 4°C for 30 minutes. Untreated tachyzoites and 0.2% saponin were used as positive and negative controls, respectively. The FACSCalibur flow cytometer was set to the PI color and configured to count 10,000 to 30,000 cells to assess the effects of gamma rays on the vitality of the tachyzoites.
3.5. Evaluation of the Infectivity Rate of Gamma-Iradiated Tachyzointes in HeLa Cell
The growth process of tachyzoites exposed to different doses of gamma rays was evaluated in HeLa cell culture. Tachyzoites exposed to different doses of gamma rays (50, 100, and 200 Gy) were transferred to HeLa cell culture flasks with 70% confluence at a 3:1 ratio. The flasks were then incubated at 37°C, > 80% humidity, and 5% CO2. After 8 hours, the culture medium was changed to remove extracellular parasites. The relevant cultures were examined under an inverted microscope, and the parasite load was recorded and compared with the control sample. In the control sample, unexposed tachyzoites were transferred to HeLa cell culture flasks. All experiments were performed in triplicate (18).
The parasite load (infectivity index) was calculated as follows: The mean number of tachyzoites in a host cell determined in 100 infected cells multiplied by the ratio of infected cells to 100 cells in the cell culture.
3.6. Animal
Inbred female BALB/c mice, aged 4 - 6 weeks and weighing 20 - 24 g, were used for in vivo experiments. The animals were housed in cages at a temperature of 22 ± 2°C with a 12-hour light/dark cycle and 40 - 50% relative humidity at the Center of Comparative and Experimental Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. During the experiments, the mice had access to water and a healthy diet.
3.7. Vaccinating BALB/ c Mice with Gamma-Irradiated Tachyzoites
1 × 105 tachyzoites of the T. gondii RH strain, treated with different doses of gamma rays, were subcutaneously injected into groups of 20 mice for each dose (50, 100, and 200 Gy). Mice were vaccinated with a single dose without a booster. Additionally, 10 mice served as the control group and were infected with 1 × 105 intact tachyzoites of the RH strain, while another 10 mice were not infected with any tachyzoites. The mice were monitored for the pathogenic process, and survival was checked daily. The mortality rate was documented for each group.
3.8. Cytokine Assay in Vaccinated BALB/c Mice
To investigate the immune response in mice vaccinated with tachyzoites exposed to different doses of gamma radiation, blood sampling was performed from 5 surviving animals per group after 2 weeks. The immune factors, including interleukins 2 and 10, and interferon-gamma, were measured in their serum (first measurement) using a commercial kit (Mouse ELISA kit, Karmania Pars Gene). Additionally, after the vaccine challenge, the immune factors were checked in all surviving mice (second measurement).
3.9. Challenging the Vaccinated Mice with Intact Wild Type Tachyzoites
To evaluate vaccine efficacy, 4 weeks after vaccination, 1 × 105 intact tachyzoites of the T. gondii RH strain were intraperitoneally inoculated into the live mice in each group. Additionally, 1 × 105 intact tachyzoites of the RH strain were injected into five intact mice as a negative control. The mice were monitored daily, and the mortality rate was documented for each group until 30 days (21).
3.10. Statistical Analysis
Statistical analyses and graphing were performed using SPSS Software version 16 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, USA). All values were expressed as the mean ± SD. For multiple comparisons in vitro, the Kruskal-Wallis test or one-way ANOVA was performed. The survival time of the in vivo study groups was compared using the Kaplan-Meier method, with statistical significance considered at P < 0.05.
4. Results
4.1. The Effect of Different Dose of Gamma Ray on Tachyzoites
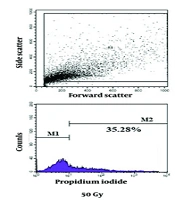
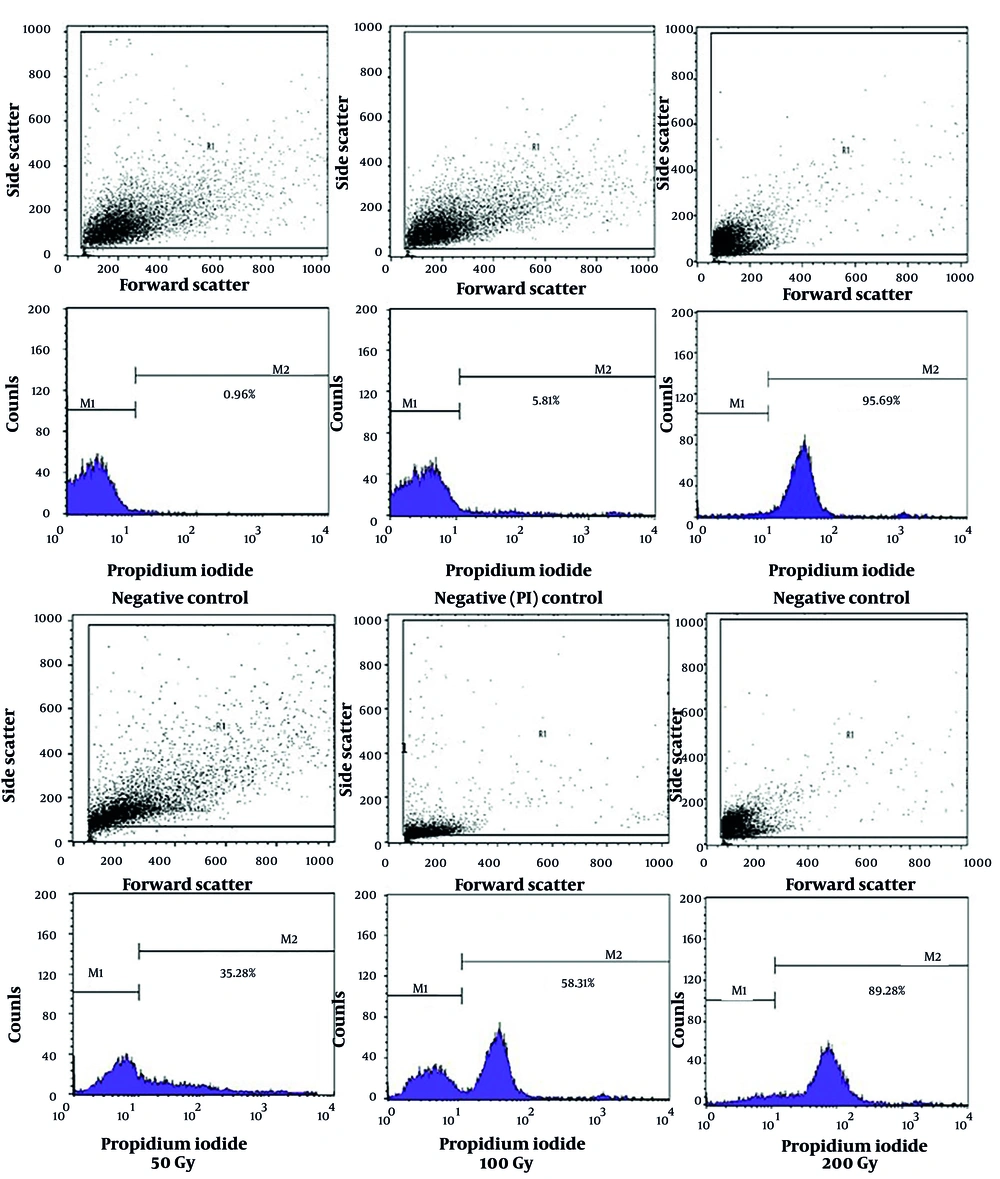
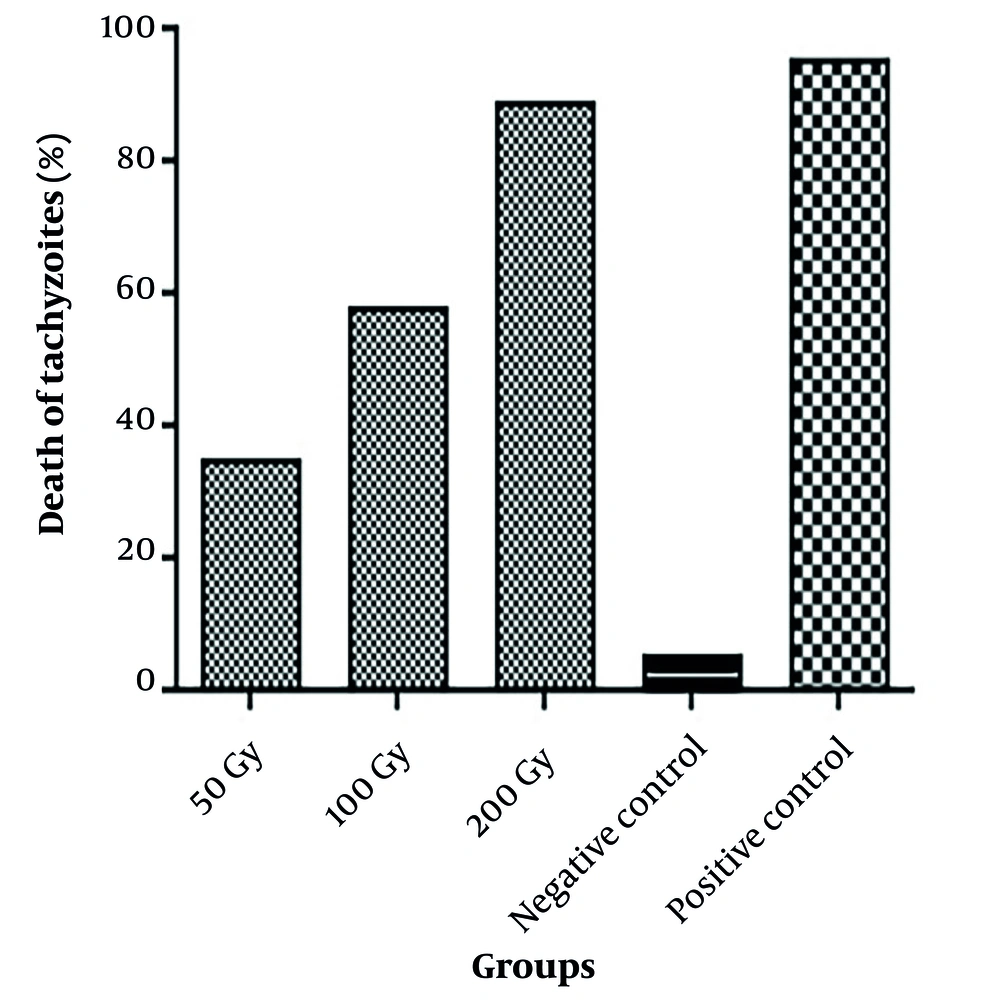
The flow cytometry results of Toxoplasma the tachyzoites exposed to different doses of gamma rays, along with control groups, are shown in Figures 1 and 2. Mortality rates of 35.28%, 58.31%, and 89.28% were observed in tachyzoites exposed to 50 Gy, 100 Gy, and 200 Gy of gamma rays, respectively. In the calibration group (parasites without PI), 0.96% mortality was observed, while the negative control (parasites with PI) showed 5.81% mortality, and the positive control exhibited 95.69% mortality of tachyzoites.
The flow cytometry results of Toxoplasma tachyzoites exposed to different doses of gamma ray, and control groups: Toxoplasma tachyzoites RH strain exposed to saponin as positive control, negative control was unexposed tachyzoites whiteout PI, PI control was unexposed tachyzoites with PI and tachyzoites exposed to different doses of gamma ray (50G, 100G, and 200G). The area of the colored part in M2 indicates the percentage of dead tachyzoites. As much as the color area shift to M2, more tachyzoites die from exposure to the treatment.
4.2. Infectivity Index of Gamma-Irradiated Tachyzoites in Cell Culture
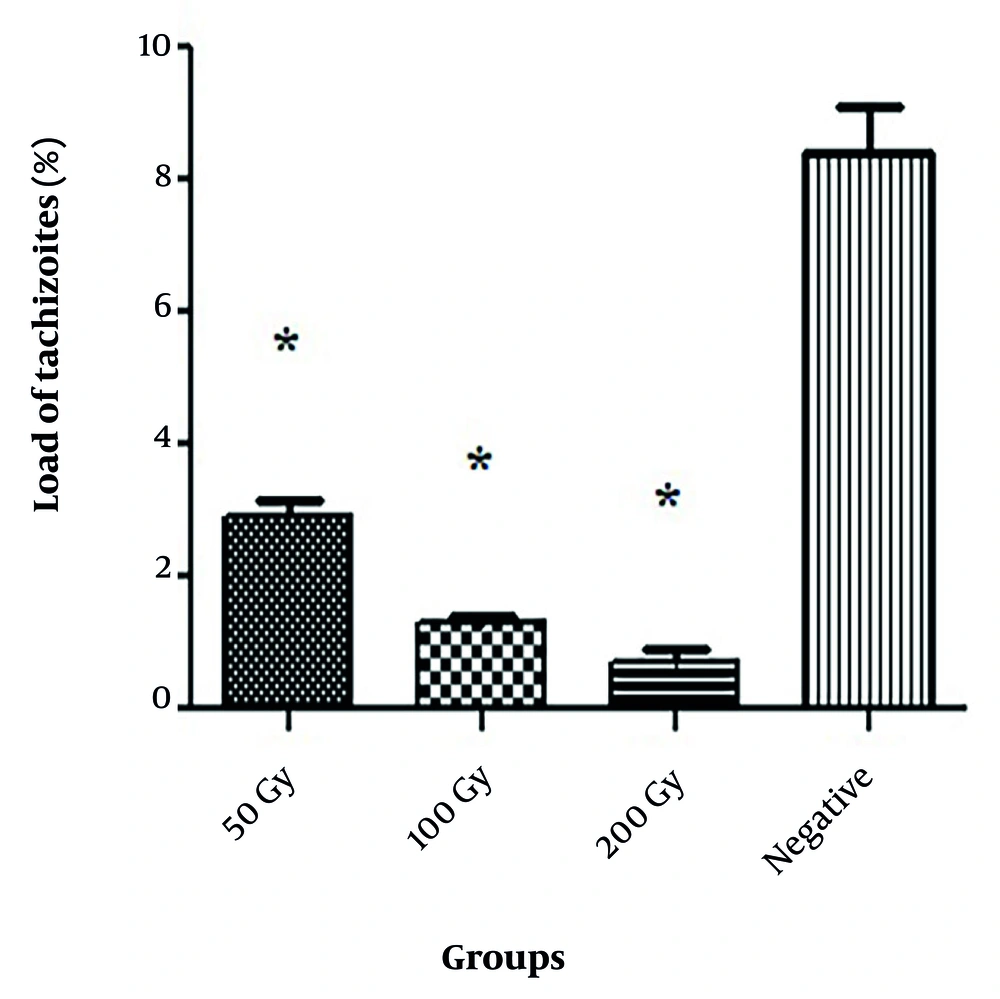
The infectivity and invasion ability of tachyzoites exposed to different doses of gamma rays were examined in the HeLa cell line using inverted microscopy (Figure 3). The load of tachyzoites in the control group in the HeLa cell culture medium was 8.4%. In the investigation of the load of RH tachyzoites exposed to different doses of gamma rays, it was found that all concentrations significantly decreased the load compared to the negative control group (P < 0.05).
4.3. In vivo Infectivity Index of Exposed Tachyzoites
To evaluate the in vivo infectivity index, the survival rate of vaccinated mice was monitored. The results showed that in the group infected with tachyzoites exposed to 50 Gy radiation, all 20 mice died 14 days after infection, with an average survival of 12.6 ± 1.17 days. In the 100 Gy group, only 4 mice died on the 18th and 19th days after infection (16 mice survived), and in the 200 Gy group, all mice survived until the start of the vaccine challenge. In the control group, death occurred from day 6 after infection with intact tachyzoites, and all mice died 8 days after infection, with an average survival of 6.8 ± 0.44 days.
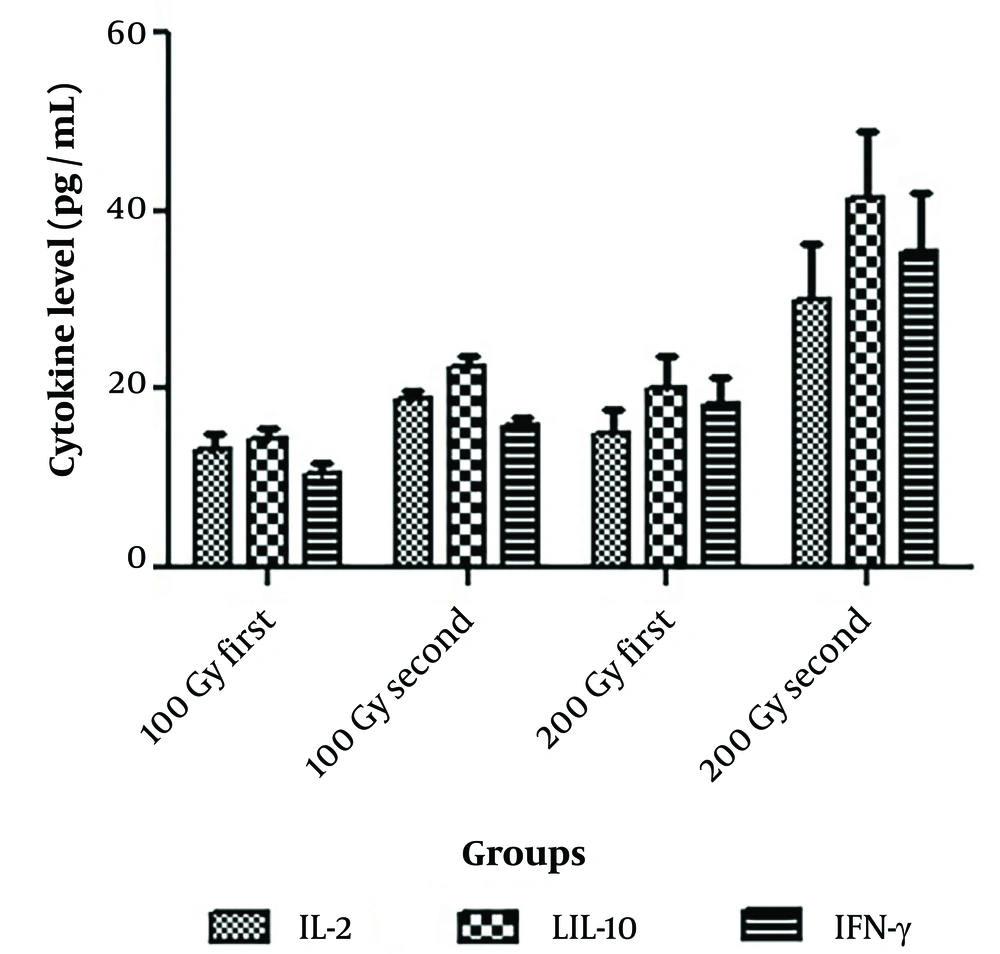
4.4. Detection of Cytokine Levels in Vaccinated Mice
Given that all mice vaccinated with a dose of 50 Gy died after two weeks, cytokine levels were investigated only at the doses of 100 Gy and 200 Gy. Blood samples were taken from 5 surviving mice in each group, and cytokine levels were measured. The ELISA results showed an increasing trend in the average levels of interleukin 2, interleukin 10, and IFN-γ between the first measurement (before vaccine challenge) and the second measurement (after vaccine challenge).
In mice treated with 100 Gy irradiated tachyzoites, the IL-10 cytokine level increased from 14.3 pg/mL ± 1.36 to 22.5 pg/mL ± 1.16 (P = 0.002). The mean IFN-γ level increased from 10.42 pg/mL ± 1.43 to 15.9 pg/mL ± 0.99 (P = 0.012), and the IL-2 level significantly increased from 13.12 pg/mL ± 2.08 to 18.81 pg/mL ± 1.14 (P = 0.021). In mice treated with 200 Gy irradiated tachyzoites, the IL-10 cytokine level increased from 20.21 pg/mL ± 3.52 to 41.54 pg/mL ± 7.34 (P < 0.001). The mean IFN-γ level increased from 18.24 pg/mL ± 3.21 to 35.34 pg/mL ± 6.83 (P < 0.001), and the IL-2 level significantly increased from 15.2 pg/mL ± 2.61 to 30.14 pg/mL ± 6.12. (Figure 4)
4.5. Vaccine Efficacy
The evaluation of the survival rate in mice was conducted over 1 - 30 days after the vaccine challenge. All mice vaccinated with irradiated tachyzoites at 50 Gy died after two weeks; therefore, the effectiveness of the vaccine was investigated only at doses of 100 Gy and 200 Gy. In the 100 Gy group, three mice died on the 14th, 21st, and 23rd days after the vaccine challenge. In the 200 Gy group, only two mice died on the 14th and 21st days after the vaccine challenge. The remaining mice survived until the end of the study. In the control group (infected with intact tachyzoites of the RH strain), death occurred from day 5 after infection, and all mice died 7 days after infection, with an average survival of 6.2 ± 0.59 days.
5. Discussion
Current treatments for toxoplasmosis, such as sulfadiazine and pyrimethamine, have limitations, including teratogenic effects, bone marrow suppression, and inefficacy against tissue cysts. Therefore, there is a critical need for an effective vaccine to prevent toxoplasmosis and mitigate its impact on public health. A successful vaccine would reduce infection rates, alleviate the burden of chronic disease, and provide a cost-effective solution for managing toxoplasmosis, especially in vulnerable populations (22, 23).
In this study, we used gamma radiation-attenuated tachyzoites of the T. gondii RH strain as a promising vaccine strategy against toxoplasmosis. The flow cytometry results clearly demonstrate that irradiation with gamma rays effectively reduces the viability and infectivity of T. gondii the tachyzoites in a dose-dependent manner. At the highest dose of 200 Gy, an impressive 89.28% of the tachyzoites were rendered non-viable. This significant reduction in viable parasites indicates that gamma radiation effectively compromises the structural and functional integrity of the tachyzoites.
The substantial decrease in the tachyzoite load in HeLa cells following gamma radiation exposure underscores the impaired infectivity and invasion capability of the irradiated tachyzoites. This reduction in infectivity is critical because it indicates that gamma radiation not only kills the parasites but also diminishes their ability to invade host cells. This dual effect of gamma radiation could be highly beneficial in controlling the spread of toxoplasmosis, especially in immunocompromised individuals who are at greater risk of severe infection.
These findings align with previous research showing the susceptibility of protozoan parasites to ionizing radiation, suggesting that gamma radiation induces lethal damage to the parasite's cellular components, leading to apoptosis or necrosis. Dubey et al. demonstrated that mice receiving oocysts exposed to doses of 0.4 and 0.2 Gy of gamma rays developed brief immunity against oocysts of a lethal parasite strain (24). In 1999, Assmar et al. reported an increase in survival rate, lymphoproliferative responses, and gamma interferon levels in mice receiving tachyzoites exposed to gamma radiation (25). Freyre et al. found that irradiating doses of 250-100 Gy of gamma rays to Toxoplasma tachyzoites allowed them to enter host cells and stimulate the immune system, but impaired their ability to reproduce and persist in host tissue (26). Zorgi et al. concluded that after irradiating cobalt-60 with a dose of 255 Gy to T. gondii tachyzoites and administering them intraperitoneally and orally to mice, CD4 cells, B cells, and CD8 cells increased cellular and humoral immune responses, contributing to the resistance of mice immunized with tachyzoites exposed to radiation (27). In 2018, da Costa et al. reported an increase in the levels of CD4, CD19, CD8, and CD3 in mice immunized with T. gondii extract proteins exposed to gamma radiation (1500 Gy) (28).
The in vivo experiments showed that BALB/c mice inoculated with 200 Gy irradiated tachyzoites exhibited complete survival when challenged with intact tachyzoites, in stark contrast to the control group, which succumbed to the infection within an average of 6.8 ± 0.44 days. The partial mortality observed in the 100 Gy group, along with the complete mortality in the 50 Gy group, underscores the importance of optimizing the irradiation dose to achieve a balance between attenuation and immunogenicity. Survival rate monitoring post-vaccine challenge revealed that higher doses of gamma radiation (100 Gy and 200 Gy) not only attenuated the tachyzoites but also preserved their ability to elicit a protective immune response in the host. These findings are promising for the development of a vaccine against toxoplasmosis, suggesting that gamma radiation-attenuated tachyzoites could provide a safe and effective means of immunization.
The increased levels of cytokines (IL-2, IL-10, and IFN-γ) in these mice further support this hypothesis. Cytokines play a pivotal role in orchestrating the immune response, and their elevated levels suggest that gamma radiation may enhance the host's ability to mount an effective immune defense against T. gondii. The observed increases in IL-2, IL-10, and IFN-γ levels post-vaccine challenge indicate a heightened immune response in mice treated with irradiated tachyzoites. IL-2 is essential for T-cell proliferation and activation (29), while IL-10 has anti-inflammatory properties that help regulate the immune response to prevent excessive tissue damage (30). IFN-γ is a key cytokine in the immune response to intracellular pathogens, including T. gondii (31). The significant increases in these cytokines suggest that gamma radiation not only kills the parasites but also modulates the immune system to enhance its effectiveness. This immunomodulatory effect could be pivotal in developing vaccines and therapeutic interventions for toxoplasmosis.
The main immune mechanism involved in resistance to T. gondii is cellular immunity (32). The primary pathway involved in this cellular immune response is the Th1 pathway, in which cytokines and interleukins are also involved (12). Toxoplasmosis infection stimulates the secretion of high levels of IL-12 and TNF-alpha, which are normally produced by macrophages. In addition to macrophages, dendritic cells are also the main IL-12-producing cells during toxoplasmosis infection. It has been shown that after incubation of T. gondii with dendritic cells in vitro, dendritic cells quickly secrete high levels of IL-12 (33). Interferon released by CD8 cells, macrophages, and NK cells can activate macrophages and enhance their ability to kill (34). By secreting IL-10, macrophages play an immunosuppressive role by inhibiting the proliferation of T lymphocytes and the synthesis of human gamma interferon and murine NK cells (35).
Evidence indicates the high importance of gamma interferon in the host's resistance to the Toxoplasma parasite (29). Therefore, the effectiveness of a vaccine against T. gondii is measured by its ability to induce immunity in the vaccinated animal, the survival of the infected animal, and the production of gamma interferon during infection. Several studies have reported a significant difference between immunized and non-immunized animal models in interferon gamma secretion and resistance to infection (28). It is also stated that the secretion of interferon gamma and IL-10 cytokines by the cell-mediated immunity (CMI) system is involved in the host's resistance against toxoplasmosis infection. High levels of interferon gamma and IL-10 have been reported in the spleen cells of vaccinated mice (36).
While the study provides promising results, several limitations need to be addressed. The long-term safety and efficacy of gamma radiation-attenuated tachyzoites in larger animal models and humans remain to be investigated. Additionally, the potential for adverse effects of high-dose gamma radiation on host tissues must be carefully evaluated. Future research should focus on elucidating the molecular mechanisms underlying the immune enhancement observed in irradiated tachyzoite-infected models and on optimizing the radiation doses to maximize therapeutic benefits while minimizing potential adverse effects.
The findings of this study have significant implications for the development of new treatments and vaccines for toxoplasmosis. The ability of gamma radiation to reduce parasite viability and enhance the immune response suggests that it could be used as an adjunct therapy to improve the efficacy of existing treatments. Additionally, the protective effects observed in the 200 Gy group indicate that gamma radiation could be explored as a standalone preventive measure or as part of a vaccine strategy.
5.1. Conclusions
In conclusion, this study demonstrates the potential of gamma radiation as an effective strategy to attenuate T. gondii and enhance the immune response in infected hosts. The significant reduction in parasite viability, impaired infectivity, and enhanced cytokine response observed in this study highlight the promise of gamma radiation in developing novel vaccines and therapeutic interventions for toxoplasmosis. These findings pave the way for further research into the use of gamma radiation in infectious disease management and the development of innovative strategies to combat toxoplasmosis and other parasitic diseases.