1. Background
Inflammatory bowel disease (IBD) encompasses a range of chronic conditions that cause inflammation of the digestive tract, including ulcerative colitis (UC) and Crohn's disease (CD). Symptoms such as abdominal pain, diarrhea, and bloody stools significantly affect the quality of life for individuals with IBD (1-3). Current treatments for IBD primarily involve drugs such as salicylic acid, glucocorticoids, immunosuppressants, and biological agents, which aim to induce or maintain symptom relief, as a complete cure remains elusive (4). The etiology of IBD is unknown; however, genetics, environment, intestinal barrier integrity, and host immunity are considered key factors that contribute to its occurrence and development (5, 6).
Research on IBD has increasingly focused on the gut microbiota (GM), an integral component of human microecology that sustains the intestinal barrier and regulates host immunity. Significant alterations in the GM structure of IBD patients are evident, primarily characterized by a decline in beneficial bacteria and an increase in pathogenic bacteria (7-9). Additionally, the intestinal flora can alter the colonic ecological environment and influence the host immune response through its metabolites. For instance, short-chain fatty acids (SCFAs) are small-molecule metabolites produced by the fermentation of dietary fiber and oligosaccharides by various symbiotic bacteria. A decrease in these bacteria in IBD patients leads to a deficiency of corresponding SCFAs in the intestinal tract, disrupting their role in regulating host immunity and intestinal inflammation (10).
However, the causal relationship between GM, the metabolites they regulate, and the onset of IBD remains unclear. Gut microbiota dysfunction is not only a cause of IBD but also a characteristic of IBD patients. The interaction between the flora and the host immune system promotes the development of intestinal inflammation and further alters the composition and proportion of the flora. Moreover, studies have shown that individuals with IBD susceptibility genes are more likely to experience dysbiosis. Mutations in IBD-related genes such as NOD2, CARD9, IRGM, and ATG16L1 affect GM signaling, leading to excessive inflammation and an increase in pathogenic bacteria (9, 11, 12).
Mendelian randomization (MR) is a genetic epidemiological method designed to infer potential causality from observed associations (13). Genetic variants can be used as instrumental variables (IVs) to estimate the causal effect of risk factors (exposure) on outcomes and reduce confounding bias (14). In recent years, MR analysis has been employed to evaluate the potential causal relationships between intestinal flora and various diseases (15-17).
2. Objectives
The present study aimed to examine the potential causal relationship between GM and IBD, as well as the mediating role of serum metabolites. Utilizing the most comprehensive genome-wide association studies (GWAS) of GM (18) and serum metabolites (19), we conducted bidirectional MR analysis to explore the causal relationship between GM and IBD. Additionally, we performed two-step MR and multivariable Mendelian randomization (MVMR) to identify potential mediating metabolites.
3. Methods
3.1. Study Design
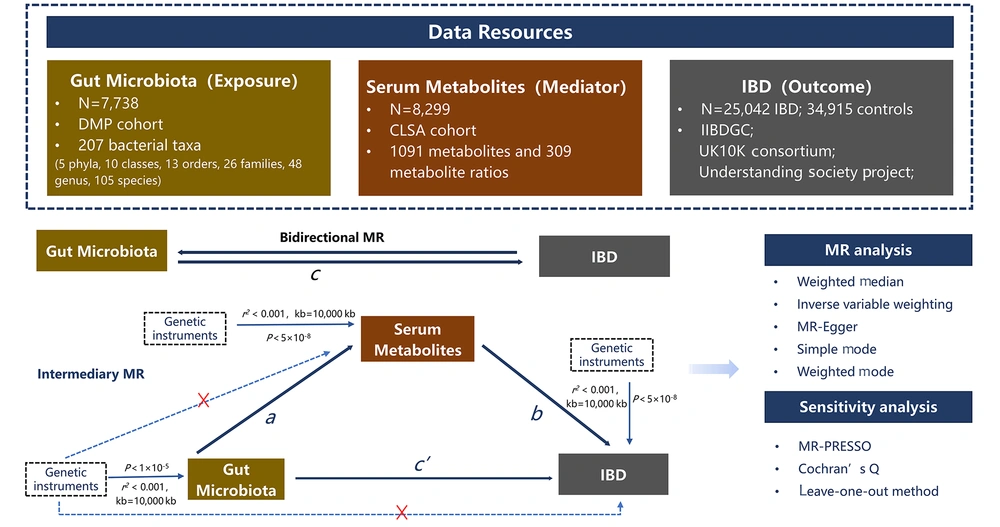
Three assumptions underlie the causal interpretation of MR estimates: (1) Single nucleotide polymorphisms (SNPs) can strongly predict exposure; (2) SNPs are irrelevant to exposure-outcome associations; and (3) SNPs affect outcomes only through risk factors. The study design is illustrated in Figure 1. We first examined the causal relationship between GM (exposure) and IBD (outcome) by conducting two-sample bidirectional MR. Next, serum metabolites were used as mediators in the intermediate analysis, and a two-step MR was employed to establish the causal pathway from GM to IBD through the mediation of serum metabolites. Finally, MVMR was conducted to validate the mediating effects of serum metabolites.
3.2. Data Sources
Summary statistics for the exposure, mediators, and outcomes were obtained from previous GWASs. An overview of the characteristics of the GWAS data is provided in Appendix 1 in Supplementary File. The abundance of human GM exposure was derived from a GWAS conducted by the Dutch microbiome project (DMP), which recruited 7,738 participants (18). The gut microbiome was determined using shotgun metagenomic sequencing, with the lowest taxonomic level in the study being the species. A total of 207 taxa were included in this study (five phyla, 10 classes, 13 orders, 26 families, 48 genera, and 105 species). We extracted GWAS statistics from the integrative epidemiology unit (IEU) database (dataset ID: “ebi-a-GCST90027651” to “ebi-a-GCST90027857”).
Data for IBD were obtained from de Lange et al.’s study (20), which conducted a GWAS of 25,305 individuals and meta-analyzed them using published summary statistics. All participants were of European ancestry, including 25,042 IBD cases and 34,915 controls from the inflammatory bowel disease genetics consortium (IIBDGC), UK10K Consortium, and understanding society project. The cases were diagnosed by accepted radiologic, endoscopic, and histopathologic evaluations, and all included cases met the clinical diagnostic criteria for IBD. The GWAS statistics for IBD were extracted from the IEU database (dataset ID: “ebi-a-GCST004131”).
We downloaded GWAS data for human serum metabolites from the GWAS Catalogue. Notably, this is the most comprehensive serum metabolite GWAS, including 1,091 metabolites and 309 metabolite ratios from 8,299 individuals in the Canadian Longitudinal Study on Aging (CLSA) cohort (19). The 850 known metabolites were involved in the metabolism of energy, amino acids, carbohydrates, lipids, nucleotides, cofactors, vitamins, peptides, and xenobiotics.
3.3. Selection of Genetic Instrumental Variants
In this study, we selected SNPs associated with the gut microbiome at a significance level of P < 1 × 10-5, based on previously published studies (17, 21). We used a genome-wide significance P-value threshold (< 5 × 10-8) to select genetic IVs for the metabolites and IBD. We selected genetic IVs for metabolites and IBD using a clumping procedure implemented in R software. This procedure ensured the independence of the IVs, retaining only SNPs with a linkage disequilibrium (LD) threshold of r2 < 0.001 and a physical distance > 10,000 kb. Additionally, we computed F-statistics using the equation F = b2/se2 for SNPs to assess their efficacy as IVs (22). The SNPs with an F-statistic > 10 were required to ensure sufficient power to limit bias from weak IVs.
3.4. Mendelian Randomization and Statistical Analysis
3.4.1. Bidirectional Mendelian Randomization Analyses
Bidirectional MR analysis was conducted using inverse variance weighting (IVW) as the primary estimation method. The IVW provides a more accurate estimate of causal effects when the IVs satisfy the three primary assumptions. Due to potential bias from pleiotropic IVs, we also applied weighted median (WM), MR-Egger, simple mode, and weighted mode methods to enhance the reliability and stability of hypothesis testing. The beta values from these four methods should align with the direction of the IVW. To improve result feasibility and conduct subsequent sensitivity analysis, we excluded bacteria or metabolites with ≤ three SNPs.
3.4.2. Sensitivity Analysis
When using IVW as the main method, it is crucial to ensure that SNPs are not pleiotropic to avoid biased results. Sensitivity analysis included tests for horizontal pleiotropy and heterogeneity. MR-Egger regression analysis was used to measure horizontal pleiotropy, with significant intercepts indicating its presence. Cochran’s Q (23) test assessed SNP heterogeneity, with statistical significance (P < 0.05) indicating significant heterogeneity. Outliers were detected using the MR pleiotropy residual sum and outlier tests (MR-PRESSO). Detected outliers were removed, and the remaining IVs were reanalyzed. In cases of heterogeneity, a random-effects model was employed for comprehensive analysis. The leave-one-out method evaluated the influence of single SNPs on MR analysis results.
3.4.3. Intermediary Mendelian Randomization
A mediation model analysis based on MR results effectively addresses confounding issues and ensures reliable causal inferences. The MVMR (24) estimates the direct impact of exposure on outcomes while controlling for mediating variables. The genetic variation of primary and secondary exposures (mediator variable) was considered as an instrument. After multivariate MR, we obtained the direct effect (c') of exposure on the outcome (c') and the regression result of the mediation of the outcome (b) in the mediation model. The indirect effect was estimated by subtracting the c' from the total effect of exposure on the outcome (c).
Two-step MR (25) uses univariate MR to estimate the influence of exposure and mediator variables on the outcome and then discusses the effect of mediator variables: (a) The causal effect of exposure on the mediating effect; and (b) the causal effect of the mediating effect on the outcome were calculated, and these two estimates (a × b) were multiplied to calculate the indirect effect. Therefore, in conducting mediated MR, we performed the following steps: (1) Univariate MR calculated the total effect of exposure on the outcome (c); (2) multivariate MR analysis obtained the c' and the effect coefficient b of the mediating variable on the outcome, or two-step MR calculated the effects a and b; (3) indirect effects were calculated.
3.4.4. Version and Name of Statistical Software
All MR analyses were performed using R software (version 4.3.1). The "TwoSampleMR", "MendelianRandomization" and "MR-PRESSO" packages were utilized.
4. Results
4.1. Genetic Instruments Included in Analysis
Based on the selection criteria, 1968 IVs were obtained from 206 bacterial isolates. The number of IVs for these bacteria ranged from 1 to 19, with a median of 9. The IBD was used as an exposure factor for the reverse MR analysis. After removing LD, 117 SNPs were selected for association with IBD. Additionally, IVs for metabolites were screened, resulting in 2736 known SNPs. The number of SNPs ranged from 1 to 11, with a median of 2. All IVs had F-statistics > 10 (Appendices 2 – 4 in Supplementary File).
4.2. Bidirectional Mendelian Randomization Analyses of Inflammatory Bowel Disease and Gut Microbiota6p
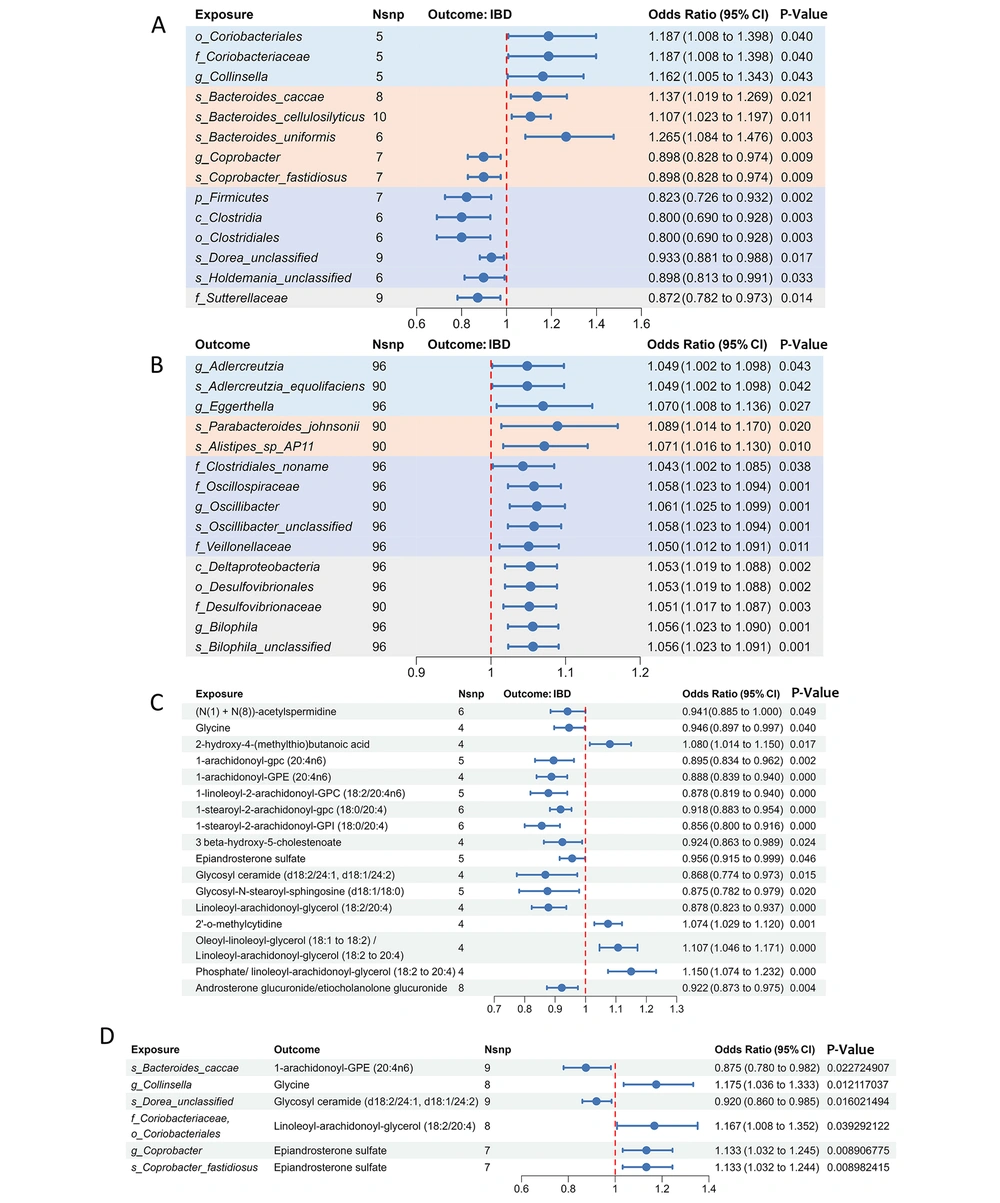
In the forward MR, the IVW analysis showed that 19 bacterial taxa were significantly correlated with IBD at the genetic level. After removing taxa with SNPs ≤ 3 and those with opposite beta values under the default five models, 14 meaningful taxa were identified for follow-up studies. One phylum, one class, one order, one family, one genus, and three species were negatively correlated with IBD, whereas one order, one family, one genus, and three species were positively correlated. Among these significant taxa, o_Coriobacteriales, f_Coriobacteriaceae, and g_Collinsella belonged to the Actinobacteria phylum, and Bacteroides_caccae, Bacteroides_cellulosilyticus, and Bacteroides_uniformis belonged to the Bacteroides genus in the Bacteroidetes phylum, all positively correlated with IBD.
The g_Coprobacter and Coprobacter_fastidiosus also belong to the Bacteroidetes phylum but are part of f_Porphyromonadaceae and were negatively correlated with IBD. f_Sutterellaceae belongs to the Proteobacteria phylum. The remaining five taxa belonged to Firmicutes, which were negatively correlated with the occurrence of IBD. Among the 14 taxa, Bacteroides_uniformis had the most pronounced effect on IBD risk (OR = 1.265, 95% CI: 1.083 - 1.476, P = 0.003) (Figure 2A and Appendix 5 in Supplementary File).
Forest plot of the causal relationship between gut microbiota (GM), serum metabolites and inflammatory bowel disease (IBD). A, the forest plot shows the causal effect of GM on IBD; B, the forest plot shows the causal effect of IBD on GM; C, the forest plot shows the causal effect of serum metabolites on IBD; D, the forest plot shows the causal effect of GM on serum metabolites. Different phyla are displayed in different specific colors. The pval,ratio and 95% confidence interval were obtained by inverse variance weighting method. OR, odds ratio; CI, confidence interval.
In assessing the causal effect of IBD on GM, it was found that the relative abundance of all taxa increased after IBD, including one class, one order, four families, four genera, and five species, which belonged to the four phyla covered by the forward MR analyses. Parabacteroides_johnsonii was most affected by IBD (OR = 1.089, 95% CI = 1.014 - 1.170; P = 0.020). Furthermore, sensitivity analyses confirmed the reliability of these results without multidirectionality or heterogeneity. A bidirectional MR study did not find a bidirectional causal relationship between any taxon and IBD (Figure 2B and Appendix 6 in Supplementary File).
4.3. Causal Associations of Serum Metabolites with Inflammatory Bowel Disease
Using the IVW method in MR analysis, 25 IBD-related variables were screened from 1021 serum metabolites and metabolite ratios. After excluding 3 variables that failed the MR-PRESSO sensitivity analysis and 5 unknown metabolites, 14 metabolites and 3 metabolite ratios were determined to be related to IBD. Among the 14 metabolites, there were 3 amino acid pathway metabolites, 10 lipid pathway metabolites, and 1 nucleotide pathway metabolite. 2-hydroxy-4-(methylthio)butanoic acid and 2'-O-methylcytidine were positively associated with IBD, whereas (N(1) + N(8))-acetylspermidine, 1-arachidonoyl-gpc(20:4n6), 1-arachidonoyl-GPE(20:4n6), 1-linoleoyl-2-arachidonoyl-GPC (18:2/20:4n6), 1-stearoyl-2-arachidonoyl-gpc (18:0/20:4), 1-stearoyl-2-arachidonoyl-GPI (18:0/20:4), 3beta-hydroxy-5-cholestenoate, epiandrosterone sulfate, glycine, glycosylceramide (d18:2/24:1, d18:1/24:2), glycosyl-N-stearoyl-sphingosine (d18:1/18:0), and linoleoyl-arachidonoyl-glycerol (18:2/20:4) were negatively associated with IBD. 1-stearoyl-2-arachidonoyl-GPI (18:0/20:4) showed the most significant protective effect against IBD (OR = 0.856, 95% CI, 0.800 - 0.916; P < 0.001). The ratios of androsterone glucuronide to etiocholanolone glucuronide, oleoyl-linoleoyl-glycerol (18:1 to 18:2) to linoleoyl-arachidonoyl-glycerol (18:2 to 20:4), and phosphate to linoleoyl-arachidonoyl-glycerol (18:2 to 20:4) were also associated with IBD (Figure 2C and Appendix 7 in Supplementary File).
4.4. Causal Relationship of "Gut Microbiome-Metabolites-IBD"
In two-step MR, 14 taxa (exposure) and 17 serum metabolites and metabolite ratios (mediators) were causally associated with IBD (outcome). A two-sample MR analysis was performed on the selected GM and serum metabolites, and the IVW results confirmed a causal relationship between only seven taxonomic units and five serum metabolites (Figure 2C and Appendix 8 in Supplementary File). The s_Bacteroides_caccae could reduce the level of 1-arachidonoyl-GPE (20:4n6), thereby increasing the risk of IBD (OR = 1.137, 95% CI 1.019 - 1.269, P = 0.021).
The g_Coprobacter and s_Coprobacter_fastidiosus shared the same IVs, both of which increased the level of epiandrosterone sulfate and played a protective role in the occurrence of IBD. The mediating effects were calculated using the product method and were found to be 12.45%, 5.56%, and 5.57%, respectively. After adjusting for intermediate metabolites using multivariate MR, the c's of flora and metabolites on IBD were calculated. The effects of s_Bacteroides_caccae, g_Coprobacter, and s_Coprobacter_fastidiosus remained significant. These results suggest a partial mediating effect between the flora, serum metabolites, and IBD.
However, the c's of g_Collinsella, s_Dorea_unclassified, o_Coriobacteriales, and f_Coriobacteriaceae on IBD were not significant after adjusting for the corresponding metabolites (P > 0.05). This result indicates that the causal relationship between these four taxonomic units and IBD was entirely mediated. Additionally, the product of the test coefficients a and b was opposite to the c', suggesting the existence of a masking effect. Glycine, glycosyl ceramide (d18:2/24:1 and d18:1/24:2), and linoleoyl-arachidonoyl-glycerol (18:2/20:4) were protective factors against IBD (Figure 2D and Table 1).
| Exposure | Mediator | Total Effect (c) a | Direct Effect (a) a | Direct Effect (b) a | Mediation Effect (a × b) a | Indirect Effect (c’) a | Indirect Effect (c’) [P-Value] | Indirect Effect (b’) a | Indirect Effect (b’) [P-Value] | Mediated Proportion [(a × b)/c] (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| s_Bacteroides_caccae | 1-arachidonoyl-GPE (20:4n6) | 0.128 (0.019 ~ 0.238) | -0.134 (-0.249 ~ -0.019) | -0.119 (-0.175 ~ -0.062) | 0.016 (-1.879 ~ 2.041) | 0.106 (0.008 ~ 0.205) | 0.034 | -0.120 (-0.176 ~ -0.065) | 0.0000 | 12.45 |
| g_Collinsella | Glycine | 0.150 (0.005 ~ 0.295) | 0.161 (0.035 ~ 0.287) | -0.055 (-0.108 ~ -0.003) | -0.009 (-1.899 ~ 2.021) | 0.130 (-0.017 ~ 0.277) | 0.075 | -0.057 (-0.104 ~ -0.010) | 0.0185 | 6.0 |
| s_Dorea_unclassified | Glycosyl ceramide (d18:2/24:1, d18:1/24:2) | -0.069 (-0.127 ~ -0.012) | -0.083 (-0.151 ~ -0.016) | -0.141 (-0.256 ~ -0.027) | 0.012 (-1.88 ~ 2.04) | -0.051(-0.112 ~ -0.010) | 0.100 | -0.135 (-0.214 ~ -0.055) | 0.0008 | 17.27 |
| o_Coriobacterialesf_Coriobacteriaceae | Linoleoyl-arachidonoyl-glycerol (18:2/20:4) | 0.171 (0.008 ~ 0.335) | 0.154 (0.008 ~ 0.301) | -0.130 (-0.195 ~ -0.065) | -0.020 (-1.898 ~ 2.022) | 0.181 (-0.044 ~ 0.407) | 0.115 | -0.137 (-0.218 ~ -0.058) | 0.0007 | 11.66 |
| g_Coprobacter | Epiandrosterone sulfate | -0.108 (-0.108 ~ -0.026) | 0.125 (0.031 ~ 0.219) | -0.045 (-0.089 ~ -0.001) | -0.006 (-1.913 ~ 2.007) | -0.091 (-0.142 ~ -0.040) | 0.0005 | -0.159 (-0.259 ~ -0.060) | 0.0017 | 5.56 |
| s_Coprobacter_fastidiosus | Epiandrosterone sulfate | -0.108 (-0.108 ~ -0.026) | 0.125 (0.031 ~ 0.218) | -0.045 (-0.089 ~ -0.001) | -0.006 (-1.913 ~ 2.007) | -0.091 (-0.142 ~ -0.039) | 0.0005 | -0.159 (-0.259 ~ -0.059) | 0.0018 | 5.57 |
a Values are expressed as β (95%CI).
5. Discussion
Patients with IBD experience dysbiosis of the GM and metabolism (10, 26, 27). However, transgenes and their metabolites in IBD patients are influenced by individual differences, disease, diet, and time of day. Clarifying causal relationships through observational studies and traditional epidemiological methods is challenging. The MR is based on genetic variants associated with exposure factors and assesses the association between these genetic variants and outcomes, thus avoiding bias due to environmental factors in observational studies. This approach provides a new perspective for studying the mechanisms of IBD.
Previous studies have used MR to explore the causal relationship between GM and IBD. Zhuang et al.’s study confirmed that OTU10032-unclassified Enterobacteriaceae was associated with a high risk of IBD (OR, 1.03; 95% CI, 1.00 - 1.06; P = 0.033) (28). Taurine, a related metabolite of Enterobacteriaceae, was positively correlated with the risk of IBD (OR, 1.04; 95% CI, 1.01 - 1.08; P = 0.016). Liu et al.’s study (29) found that six genera of bacteria were associated with the risk of IBD, UC, or CD. Eubacterium ventriosum had a lower risk of IBD, whereas Coprococcus 2 had a higher risk. Moreover, f_Verrucomicrobiaceae, g_Akkermansia, and g_Dorea were confirmed to have a causal relationship with IBD (30).
The innovation of this study is the use of GWAS data, which contained species-level GM, and the most comprehensive metabolite GWAS data as an intermediary variable to explore the causal relationship between GM and IBD. This study found that 14 taxa were causally associated with IBD. The results of the reverse MR analysis suggested an increase in the abundance of 15 bacteria in patients with IBD. o_Coriobacteriales (OR = 1.187, 95% CI: 1.008 - 1.398, P = 0.040), f_Coriobacteriaceae (OR = 1.187, 95% CI: 1.008 - 1.398, P = 0.040), and g_Collinsella (OR = 1.162, 95% CI: 1.005 - 1.343, P = 0.042) were risk factors for IBD. Alam et al. sequenced the feces of 20 patients with IBD (11 with CD and 9 with UC) and 10 healthy volunteers. Compared to healthy individuals, the abundance of Burkholderiaceae and Coriobacteriaceae in IBD patients increased, confirming the conclusions of our study (31). The g_Collinsella, derived from Coriobacteriaceae, is a ubiquitous bacterium in the human intestine that can produce hydrogen, SCFAs, and lactic acid. A high abundance of Collinsella correlated with a positive response to anti-tumor necrosis factor therapy, implying a connection between these bacteria and the pathological innate inflammatory pathway (32). The increase in Collinsella was uniquely associated with the likelihood of severe penetrating disease in a pediatric CD cohort (33).
Further mediated MR results confirmed that g_Collinsella increased serum glycine levels, while o_Coriobacteriales and f_Coriobacteriaceae increased serum linoleoyl-arachidonoyl-glycerol (18:2/20:4) levels. After multivariate MR analysis, the causal relationship between g_Collinsella, o_Coriobacteriales, and f_Coriobacteriaceae and IBD disappeared, whereas a causal relationship between metabolites and IBD still existed. This suggests that the risk effect of bacteria on IBD may be caused by other genetic-related pathways, but the increase in serum glycine and linoleoyl-arachidonoyl-glycerol (18:2/20:4) has a protective effect on IBD. Glycine has antioxidant properties that scavenge free radicals and attenuate oxidative stress damage. In IBD, intestinal inflammation may lead to elevated levels of oxidative stress, and the antioxidant effect of glycine may help mitigate this damage (34). Additionally, glycine may reduce the production of inflammatory mediators by affecting cyclooxygenase activity in the arachidonic acid metabolic pathway, thereby alleviating IBD symptoms to some extent (35).
Metabolites generated by arachidonic acid metabolism via the cyclooxygenase and lipoxygenase pathways, such as prostaglandins and leukotrienes, play an important role in regulating the inflammatory response in the intestine (36). Arachidonic acid metabolites are involved in regulating innate immune function in the gut, influencing the development and differentiation of immune cells in the intestinal epithelial barrier and lamina propria (37). Studies suggest that the mechanism of arachidonic acid metabolites in intestinal inflammation may involve interactions with endogenous cannabinoid metabolism. COX-2 metabolizes not only arachidonic acid but also endogenous cannabinoids to produce biologically active lipids such as prostaglandin glycerol esters and prostaglandin ethanol amides (38, 39). Therefore, the specific mechanisms linking these metabolites to inflammation in IBD may involve inflammatory responses, oxidative stress, immune dysregulation, and the intestinal barrier.
Bacteroides are the most common and abundant members of human intestinal microflora. Several metabolic activities are performed by Bacteroides in the human colon, including carbohydrate fermentation, nitrogen oxidation, and bile acid metabolism (40). In addition to preventing infection by potential pathogens, Bacteroides can produce SCFAs (41). Studies have shown that s_Bacteroides_uniformis can reshape the composition of colon intestinal flora, regulate the metabolism of colon lipids and bile acids, and regulate the NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways by inhibiting the IL-17 signaling pathway, thus improving the development of DSS-induced colitis. The s_Bacteroides_uniformis or bile acid supplementation has proven to be a potential therapy for colitis and other diseases associated with intestinal barrier dysfunction (42).
Our study found that the causal effects of p_Bacteroidetes on IBD varied depending on the bacterial genus. s_Bacteroides_cellulosilyticus, s_Bacteroides_caccae, and s_Bacteroides_uniformis in g_Bacteroides were risk factors for IBD. Bacteroides_uniformis had a pronounced effect on IBD risk (OR = 1.265, 95% CI: 1.083 - 1.476, P = 0.003). The s_Bacteroides_caccae could reduce the level of 1-arachidonoyl-GPE (20:4n6), thereby increasing the risk of IBD (OR = 1.137, 95% CI 1.019 - 1.269, P = 0.021). However, g_Coprobacter and s_Coprobacter_fastidiosus were protective factors against IBD, increasing the level of epiandrosterone sulfate and playing a protective role in the occurrence of IBD. Reverse MR analysis confirmed that IBD increased the abundance of s_Alistipes_sp_AP11 and s_Parabacteroides_johnsonii.
Enterococcus faecalis, as a probiotic, is considered to have a potential protective effect against IBD. This is due to its ability to stimulate the secretion of the anti-inflammatory cytokine IL-10 from dendritic cells, an important anti-inflammatory mediator that inhibits the production of other pro-inflammatory cytokines in the gut, such as IL-12 and INF-γ (43). Additionally, E. faecalis can enhance intestinal barrier function and modulate the immune response by producing SCFAs such as butyrate (44). Enterococcus faecalis may also indirectly influence the development and progression of IBD by affecting the gut-hepatic axis and regulating bile acid metabolism (45). The metabolite of E. faecalis, epiandrosterone sulfate, may exert a protective effect by modulating the host’s immune system. Epiandrosterone sulfate is known to have anti-inflammatory and immunomodulatory effects, capable of attenuating the inflammatory response in IBD by inhibiting the production of pro-inflammatory cytokines and promoting the release of anti-inflammatory cytokines (46).
Various probiotics in the phylum Firmicutes, such as lactobacilli, Eubacterium, and Ruminococcus, have been shown to improve intestinal inflammation through various pathways. Selenium-enriched Lactobacillus has been found to significantly alleviate colitis and liver inflammation induced by DSS. It reduces oxidative stress in colon tissue and exerts its therapeutic effect by regulating the NF-κB-P65 signaling pathway and the structure of the intestinal microflora (47). Ruminococcus abundance increases with active IBD, from an average of 0.1% in healthy controls to 69% in IBD patients (48). Sequencing of the colonic tissues of patients with CD and healthy controls revealed that the enrichment degree of Ruminococcus in patients was significantly improved (49).
This study confirmed that s_Dorea_unclassified and s_Holdemania_unclassified belonging to Firmicutes were protective factors against IBD. s_Dorea_unclassified may affect the occurrence of IBD by regulating the level of glycosyl ceramide (d18:2/24:1, d18:1/24:2); however, this protective effect disappeared after adjusting for mediator metabolites in MVMR, suggesting the existence of other potential pathways. Wang et al. suggested that Dorea is highly expressed in patients with IBD, has pro-inflammatory effects, and is positively correlated with waist circumference, body mass, and diastolic blood pressure (50). However, Bajaj et al. confirmed a decrease in the abundance of Dorea in IBD patients (51). Additionally, the role of s_Holdemania_unclassified in the pathogenesis of IBD has not yet been identified and may be a potential focus for follow-up studies.
In recent years, there has been an increase in research on the role of GM in diseases. However, the study of GM is a dynamic and long-term process, and interactions among hosts, diseases, and microbiota can lead to differences in research results, affecting the progress of disease research. The clinical efficacy of probiotic supplementation and fecal microbiota transplantation did not meet expectations. A MR study that explored the causal relationship between GM and disease based on genetic variation has provided new insights. When studying the pathogenesis of IBD, attention should be paid to the GM and microbial metabolites that cause the onset of IBD, rather than the microbiota affected by IBD, which may yield more meaningful results.
Nevertheless, our study has some limitations. First, both the patients and controls were Europeans. Rehman’s study (52) found that the microbiota associated with IBD is shared among populations, as well as privately owned, revealing that the GM associated with IBD is influenced by disease status and geographical factors. Thus, our study may have limitations when extended to other ethnic groups (31). Second, owing to the large amount of data, we did not analyze GM and specific subtypes of IBD, which requires further research. Third, to obtain sufficient GM, we selected IVs (P < 1 × 10-5) from GM that were significantly higher than the traditional whole-genome levels (P < 5 × 10-8).
5.1. Conclusions
In summary, this study assessed the potential causal role of the GM in IBD, as well as the mediating role of metabolites. These findings provide new insights into possible therapies for IBD and offer valuable clues for pathogenesis studies.