1. Background
One of the most important aspects of a parasite is its ability to use its host for feeding, transport, shelter, and reproduction. Based on this, according to the behavioral manipulation hypothesis, it is known that parasites tend to increase their infection rates by causing behavioral changes in their hosts (1). One of these parasites, Toxoplasma gondii, has been reported to change its host’s behavior by altering neurotransmitter levels in the brain. For example, it has been observed that infected rats do not respond to cat odor and, as a result, their fear of cats is reduced (2). Toxoplasma gondii is an obligate intracellular protozoan of the phylum Apicomplexa. It infects many vertebrates, including humans, and poses a significant health problem. Toxoplasmosis is estimated to affect about one-third of the world’s population (2, 3).
In the acute phase of toxoplasmosis, the immune system controls the spread of the parasite in the body and relieves the symptoms of the disease. In the chronic phase of the disease, the parasite persists in the infected host for life by transforming into latent tissue cysts within muscle and neural cells. The course of the disease varies according to the immune status of the host (4, 5). In chronic toxoplasmosis, T. gondii bradyzoites can cause many neurotransmitter changes in the brain. For example, they can significantly increase dopamine, a neurotransmitter, by affecting dopaminergic neurons. Toxoplasma gondii can also affect glutamate, serotonin, adrenaline, and noradrenaline levels in the brain (6). Toxoplasma gondii tissue cysts have been reported to be a risk factor for encephalopathy, epileptic seizures, schizophrenia, anxiety, and depression, depending on their location in the brain (7). It has also been reported that latent toxoplasmosis increases the risk of individuals smoking and becoming addicted to other substances. It is estimated that the increased risk may be related to the effects on dopaminergic pathways (6), and studies on this subject continue.
2. Objectives
3. Methods
3.1. Study Design
This cross-sectional study was designed with a patient and control group, and conducted at the Van Yüzüncü Yıl University Faculty of Medicine Parasitology Laboratory between January 2022 and December 2023. The patient group included smokers who applied to the Alcohol and Substance Addiction Treatment and Education Unit of the Health Sciences University (SBU) Van Training and Research Hospital. Individuals with any substance addiction other than smoking were excluded from the study. The control group included volunteers who applied to any outpatient clinic of the SBU Training and Research Hospital and were directed to the biochemistry laboratory. They declared that they had not used any substances in the last year. Individuals who did not volunteer to participate in the study and those who were diagnosed with any diseases, such as cancer, diabetes, hemodialysis, epilepsy, schizophrenia, and Parkinson’s, were not included in the study.
3.2. Sample Size and Data Collection
The sample size was calculated using G*Power 3.1.9.7 Analysis software within the scope of chi-squared goodness-of-fit tests (10). In the calculations, a minimum of 50 samples in each group was determined when the Power was 0.80, the effect size was 0.4, and the type 1 error was 0.05. In order to increase the power of the test, 90 substance addicts were included in the patient group, which was higher than the value determined in the calculations. The control group included 82 patients: Patient blood samples were taken from the biochemistry laboratory, and the serum was separated and brought to the parasitology laboratory. The patients' age and sex information was obtained from the hospital automation system.
3.3. Determination of Toxoplasma gondii Seroprevalence
The presence of anti-T. gondii immunoglobulin G (IgG) antibody in the serum was determined using DRG enzyme-linked immunosorbent assay (ELISA) IgG kits (DRG Instruments GmbH, Marburg, Germany), following the manufacturer’s instructions. First, one of the ELISA plate wells was designated as the blank. Next, 100 µL of the standards, positives, negative controls, and samples were added into selected wells. The wells were then incubated for 60 min at 37°C. Next, the contents of the wells were removed and then washed 5 times with a wash solution. As the next step, 100 µL of the enzyme conjugate was added into each well, except the blank well, and incubated for 30 min at room temperature. Then, the contents of the wells were removed, and the same wash cycle was repeated. Next, 100 µL of tetramethylbenzidine substrate solution was added to all the wells and incubated for exactly 15 min at room temperature in the dark. To stop the reaction, 100 µL of stop solution was added. The absorbance of the plate wells was read at 450 - 620 nm with a plate reader within 5 min. For interpretation of the quantitative results, IgG antibody levels greater than 55 IU/mL were considered positive.
3.4. Determination of the Cortisol and Melatonin Levels in the Blood Serum
After determining T. gondii positivity, the smokers were divided into two groups, T. gondii positive and T. gondii negative, and the cortisol and melatonin levels of the two groups were compared. Cortisol and melatonin levels in the blood serum samples were evaluated using Sunredbio Human Dopamine ELISA Kits (Shanghai Sunred Biological Technology Co., Ltd., Baoshan, Shanghai, China). This was done according to the instructions provided by the manufacturer.
3.5. Statistical Analysis
The Z test and Fisher’s exact test were used to determine statistical significance at P < 0.05. The Independent-Samples Kruskal-Wallis t-test was used to determine the relationship between the cortisol and melatonin levels and T. gondii positivity since the values did not show normal distribution. IBM SPSS Statistics for Windows 26.0 (IBM Corp., Armonk, NY, USA) and Minitab 14 were used for statistical analysis.
4. Results
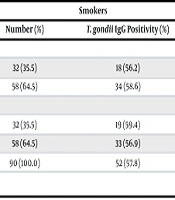
While 58 (64.4%) of the smokers were male and 32 (35.6%) were female, 33 (39%) of those in the control group were male and 49 (61%) were female. The mean age of the smokers was 32.6 ± 10.9 (range: 19 to 72) years, and that of the controls was 42.4 ± 18.2 (range: 19 to 79) years. Toxoplasma gondii IgG was detected in 52 (57.8%) of 90 smokers and 23 (28%) of 82 those in the control group (Table 1). A statistically significant relationship was found between T. gondii positivity and smoking addiction (P = 0.001). In the T. gondii-positive smokers, the mean cortisol level was 45.2 ± 23.7 mcg/dL, and the mean melatonin level was 53.1 ± 50.2, while in the T. gondii-negative smokers, the mean cortisol level was 42.3 ± 21.7 and the mean melatonin level was 48.3 ± 34.5. In the statistical evaluation, no relationship was found between T. gondii positivity and cortisol and melatonin in smokers (Table 2).
| Groups | Smokers | Control Group | P-Value | ||
|---|---|---|---|---|---|
| Number (%) | T. gondii IgG Positivity (%) | Number (%) | T. gondii IgG Positivity (%) | ||
| Gender | |||||
| Female | 32 (35.5) | 18 (56.2) | 49 (59.8) | 14 (28.6) | 0.011 |
| Male | 58 (64.5) | 34 (58.6) | 33 (40.2) | 9 (27.3) | 0.001 |
| Age | |||||
| ≤ 35 | 32 (35.5) | 19 (59.4) | 38 (46.3) | 8 (21.0) | 0.001 |
| > 35 | 58 (64.5) | 33 (56.9) | 44 (53.7) | 15 (34.1) | 0.018 |
| Total | 90 (100.0) | 52 (57.8) | 82 (100.0) | 23 (28.0) | 0.001 |
Toxoplasma gondii Immunoglobulin G Positivity Distribution by Age and Sex
| Variables | Toxoplasma gondii | Mean ± SD | SEM | P-Value |
|---|---|---|---|---|
| Cortisol | Positive | 45.2 ± 23.7 | 4.8 | 0.597 |
| Negative | 42.3 ± 21.7 | 2.8 | ||
| Melatonin | Positive | 53.1 ± 50.2 | 10.2 | 0.615 |
| Negative | 48.3 ± 34.5 | 4.4 |
Cortisol and Melatonin Levels in Smokers
5. Discussion
All living things, including parasitic agents, are programmed for the continuation of their species. In some studies, it has been reported that parasitic agents cause some behavioral changes in the hosts and intermediate hosts they settle in to increase their spread or ensure the continuation of their species. The desire of mice infected with T. gondii to stay close to cats, which are their hunters, even though they should run away from them, is due to toxoplasmosis settling in the host brain and causing behavioral changes. Although numerous studies have examined T. gondii in various patient groups, research on its impact on human behavior remains limited (11). In the current study, a relationship was found between smoking and T. gondii seropositivity. In other words, it can be said that T. gondii directs the host toward smoking. However, despite the limited number of studies, one, like the current study, has reported a positive relationship between T. gondii seropositivity and smoking (12). On the contrary, there are studies reporting a negative correlation between T. gondii seropositivity and smoking (6, 13, 14).
Alvarado-Esquivel et al. (12) attributed the positive correlation between T. gondii seropositivity and smoking to the increased risk of tobacco users contracting T. gondii infection as a result of transferring the parasite from their hands to their mouths while smoking. Bahreini et al. (6), who reported a negative correlation, claimed that since smoking and T. gondii have parallel effects on the same neurotransmitters, such as dopamine, the desire of a person infected with toxoplasmosis to smoke may decrease. However, in the current study, contrary to the claims of Bahreini et al. (6), the T. gondii seroprevalence in smokers was high. A meta-analysis study conducted by Sutterland et al. (10) revealed a positive correlation between substance addiction and T. gondii infection. Their results support those of the present study, in which a relationship was found between smoking addiction and T. gondii infection, but the molecular pathways of this relationship need to be elucidated.
The primary addictive substance in cigarettes is nicotine, which, when inhaled with smoke, reaches the brain and stimulates dopaminergic neurons in the reward system. Thus, it triggers the release of dopamine, an important component of addiction (15). Melatonin reduces nicotine-seeking behavior by affecting the dopaminergic pathway (9). Another hormone investigated in the current study, cortisol, is a stress hormone that can be affected by smoking and is known to be associated with moderately elevated cortisol levels. In addition, total cortisol (TC) levels are known to increase within the first 20 min after smoking. On the other hand, acute nicotine withdrawal has been shown to cause a decrease in TC levels (16).
As mentioned above, dopamine, melatonin, and cortisol have an important place in smoking addiction. It is known that T. gondii infection affects the dopaminergic pathway (6), but no studies could be found in the literature on the effect of T. gondii infection on melatonin and cortisol levels. In the present study, the mean levels of both melatonin and cortisol were high in the T. gondii-positive smokers, but the difference between these levels when compared to the T. gondii-negative smokers was not statistically significant. This study had several limitations. First, the study sample was collected from a single center. The prevalence of smoking addiction in societies may be affected by cultural differences in the population, which may also affect the results of a single-center study. Another limitation was that the blood cortisol levels of the participants were measured. Cortisol can be measured in various body fluids, but it is usually measured in saliva, and cortisol levels in individuals may vary depending on the time of day.
5.1. Conclusions
In conclusion, we believe that there is an association between T. gondii infection and smoking addiction, but this association is independent of melatonin and cortisol levels. Researchers should investigate the effects of T. gondii on different hormones or neurotransmitters to determine the mechanisms of the role of T. gondii in smoking addiction.