1. Background
Malaria is an endemic infectious disease caused by parasitic protozoa belonging to the genus Plasmodium, making it one of the most serious protozoan infections globally (1, 2). Various species of Plasmodium, including Plasmodium malariae, P. vivax, P. falciparum, P. ovale, and P. knowlesi, cause malaria in humans (3). This disease is transmitted to humans through the bite of female Anopheles mosquitoes (4). Anopheles mosquitoes require warm temperatures for their growth and survival. Therefore, malaria prevalence is higher in tropical and subtropical countries, and its spread depends very much on climate change (5).
Globally, in 2023, the number of malaria cases was estimated at 263 million, with an incidence of 60.4 cases per 1000 population at risk. This is an increase of 11 million cases from the previous year and a rise in incidence from 58.6 cases per 1000 population at risk in 2022. The WHO Eastern Mediterranean region has experienced a 57% increase in incidence since 2021, rising to 17.9 cases per 1000 population at risk in 2023. The top five countries carrying the heaviest estimated burden of malaria cases in 2023 were (1) Nigeria (26%), (2) the Democratic Republic of the Congo (13%), (3) Uganda (5%), (4) Ethiopia (4%), and (5) Mozambique (4%) (3).
Iran is classified as an endemic country for malaria, with a significant prevalence observed in the provinces of Sistan-Baluchistan and Hormozgan, located in the southern and southeastern regions. These two provinces alone account for more than four-fifths of reported malaria cases in the country (6). Consequently, planning to eliminate malaria in the “Horizon Program of 2025” has been on the agenda of the Iranian Ministry of Health since the beginning of 2010. The plan's approach focused on action by targeting malaria foci centers (7). The program showed promising results, as the recorded cases in 2015, 2016, and 2017 significantly dropped to less than 200, 90, and 89 indigenous cases respectively (8-11). Furthermore, malaria transmission in the southern and southeastern regions of Iran decreased (12), resulting in the absence of indigenous cases in 2018 and 2019 (13). A study conducted in the city of Khash (with a population of 130,000), located in Sistan-Baluchistan province, showed that between 1995 and 2016, 5015 malaria cases were identified.
The highest number of cases occurred in 1995, with 846 patients, while the lowest number was recorded in 2016, with only one patient (14). However, the neighborhood of Sistan-Baluchistan province with malaria-endemic countries and the continued risk of importing malaria cases from Pakistan and Afghanistan has created a great challenge politically, socially, operationally, and technically to eliminate malaria in Iran (13). Annually, P. vivax causes about 14.3 million malaria cases with clinical signs worldwide (15).
Chloroquine has been used to treat P. vivax malaria for over 60 years. Australian soldiers returning from Papua New Guinea first reported P. vivax resistance to chloroquine in a report in 1989 (16, 17). There have been reports of P. vivax resistance to chloroquine in many endemic areas (17). A study conducted in 2012 on 270 symptomatic P. vivax malaria patients in Sistan-Baluchistan province, Iran, showed that chloroquine was still effective against P. vivax at the usual therapeutic dose of 25 mg/kg body weight (18). Nateghpour et al. and Hamedi et al.'s studies in 2007 and 2002 have also confirmed the susceptibility of P. vivax to chloroquine (19, 20).
2. Objectives
The purpose of this study is to examine the malaria situation and morbidity in Sistan-Baluchistan province (southern Iran) from 2016 to September 2024. Assessing the malaria status based on demographic, epidemiological, and social variables and suggesting possible ways to manage it were the objectives of the study. Investigating the aforementioned variables in malaria hotspots can be effective in controlling the disease.
3. Methods
3.1. Study Design
A retrospective longitudinal study was employed to determine the malaria prevalence in Sistan-Baluchistan province, which ranks as the second-largest province in Iran. The province is located in the southeast of Iran, with an 1100-kilometer border with Afghanistan and Pakistan (21) and has a population exceeding 3 million people. From a socioeconomic perspective, the province is among the poorest regions in Iran (22). Owing to the vastness of the province and the high dispersion of its population, healthcare services are provided by three medical universities: Zahedan University of Medical Sciences (ZAUMS) serving Zahedan, Saravan, Sib-Soran, and Mirjaveh; Iranshahr University of Medical Sciences (IRSHUMS) covering Iranshahr, Rask, Dashtiari, Mehrestan, Sarbaz, Chabahar, Qasr-e-Qand, Nikshahr, Bampour, Fanuj, and Kanarak; and Zabol University of Medical Sciences (ZBMU) responsible for Zabol, Zahak, Hirmand, Nimroz, and Hamon.
3.2. Study Population
The source population of the study included all patients who came to selected health centers for treatment during the study periods at the health centers of ZAUMS, IRSHUMS, and ZBMU from March 20, 2016, to September 30, 2024. The inclusion criteria were all symptomatic or febrile patients visiting governmental health institutions for treatment during the study period. The study sample was selected using the census method, which involved enrolling all individuals diagnosed with malaria and registered at the health centers, resulting in a sample size of 8389 malaria patients.
3.3. Data Collection
The data was collected through the malaria registration form designed by the Ministry of Health and Medical Education (MOHME) of Iran. This study examined various factors, including demographics (such as age, gender, occupation, and place of residence), hospitalization history, previous infection history, travel history, parasitology (type of parasite), and epidemiological variables (type of disease transmission). Complete data for all patients were not available for certain variables. Specifically, data on hospitalization history and previous infection history were accessible only from one university.
3.4. Statistical Analysis
Data was entered and analyzed using SPSS 25. Descriptive statistics were used to describe the prevalence, frequency, and proportion of the study participants concerning different factors. To determine the association between gender and place of the outbreak with parasite type and care received by malaria patients, the chi-square test was used. A 95% confidence interval and P-value less than 0.05 were considered statistically significant.
4. Results
4.1. Socio-demographic and Epidemiological Characteristics of the Study Participants
According to the results, from 2016 to 2022, approximately 22,079 cases of malaria were diagnosed by the healthcare system in the southeast of Iran. The majority of these cases (77.74%) were reported in Iranshahr, located in the southernmost region of Iran. Additionally, 80.4% of malaria cases occurred in individuals of Iranian nationality, 16.5% were Pakistanis, and 3.1% were Afghans. An overwhelming majority of patients (94.2%) had no prior history of malaria infection. The highest occurrence of the disease was observed in the age groups of middle-aged and young individuals, accounting for 49.5% (4153) and 31.9% (2675) of cases, respectively.
Furthermore, the incidence of malaria was higher in males (87.1% vs. 12.9%). 44.9% (9918 of 22,079) of the patients were border drivers, who regularly commute across Iran's border as part of their occupation (Table 1). Based on Table 1, 71.7% of the patients were infected with the P. vivax type of malaria parasite. Furthermore, 64.1% of the malaria cases were imported from abroad (Pakistan). Also, about 78.8% of patients were diagnosed within the first three days of symptom onset, and treatment had commenced for them. It is worth noting that no cases of malaria were reported at ZBMU, located in the northern province, during the study period.
| Variables | No. (%) |
|---|---|
| Age group (y) | |
| Child (under 5) | 755 (3.4) |
| Teenager (6 - 18) | 4703 (21.3) |
| Young (19 - 29) | 8550 (38.7) |
| Middle-aged (30 - 59) | 7226 (32.7) |
| Elder (> 60) | 845 (3.8) |
| Sex | |
| Male | 19223 (87.1) |
| Female | 2856 (12.9) |
| Service provider university | |
| ZAUMS | 4921 (22.3) |
| IRSHUMS | 17158 (77.7) |
| Occupation | |
| Student and child a | 4864 (22.0) |
| Farmer and rancher | 670 (3.0) |
| Housekeeper | 2093 (9.5) |
| Border driver | 9918 (44.9) |
| Freelance | 4104 (18.6) |
| Employee | 430 (1.9) |
| Nationality | |
| Iran | 17757 (80.4) |
| Pakistan | 3626 (16.5) |
| Afghanistan | 693 (3.1) |
| Hospitalization history b | |
| Yes | 953 (6.1) |
| No | 14630 (93.9) |
| Previous infection history b | |
| Yes | 1063 (5.8) |
| No | 17004 (94.2) |
| Travel history b | |
| No | 6863 (35.4) |
| Yes (inside the country) | 98 (0.5) |
| Yes (Pakistan) | 12422 (64.1) |
| Type of malaria parasite | |
| Plasmodium vivax | 15837 (71.7) |
| Plasmodium falciparum | 5872 (26.6) |
| Mix species | 370 (1.7) |
| Type of disease surveillance | |
| Active c | 7389 (33.5) |
| Passive d | 14690 (66.5) |
| Epidemiology category | |
| Imported e | 17347 (78.6) |
| Introduced f | 3244 (14.7) |
| Indigenous g | 1488 (6.7) |
| Symptoms to diagnosis and treatment (day) | |
| 1 | 4730 (21.4) |
| 2 | 6145 (27.8) |
| 3 | 6533 (29.6) |
| > 3 | 4671 (21.2) |
Abbreviations: ZAUMS, Zahedan University of Medical Sciences; IRSHUMS, Iranshahr University of Medical Sciences.
a People who are under five years old or are in teaching period.
b Missing data.
c Patient identification through active services.
d Patient identification through inactive services or patient self-referral.
e Cases infected abroad.
f Cases infected through contact with an imported case.
g Cases lacking evidence of importation or direct association with an imported case.
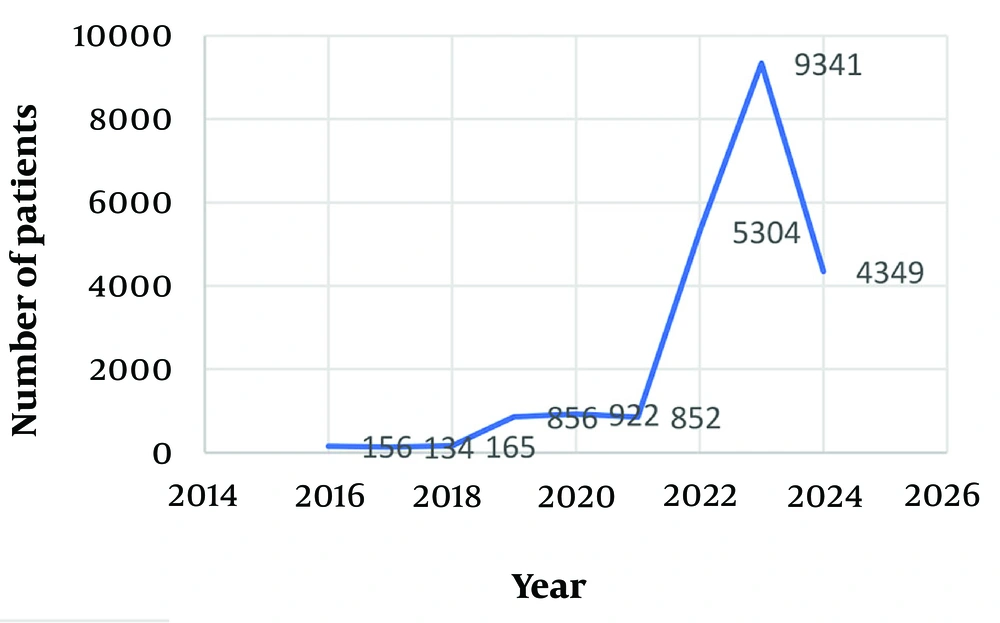
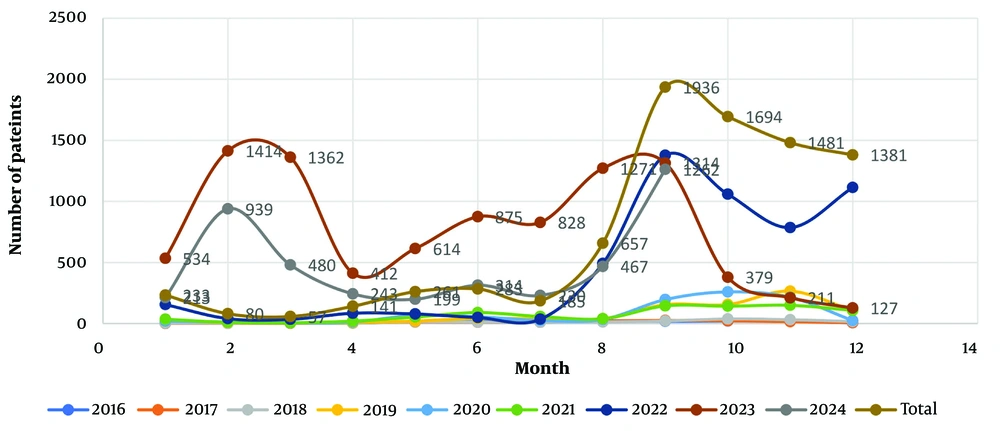
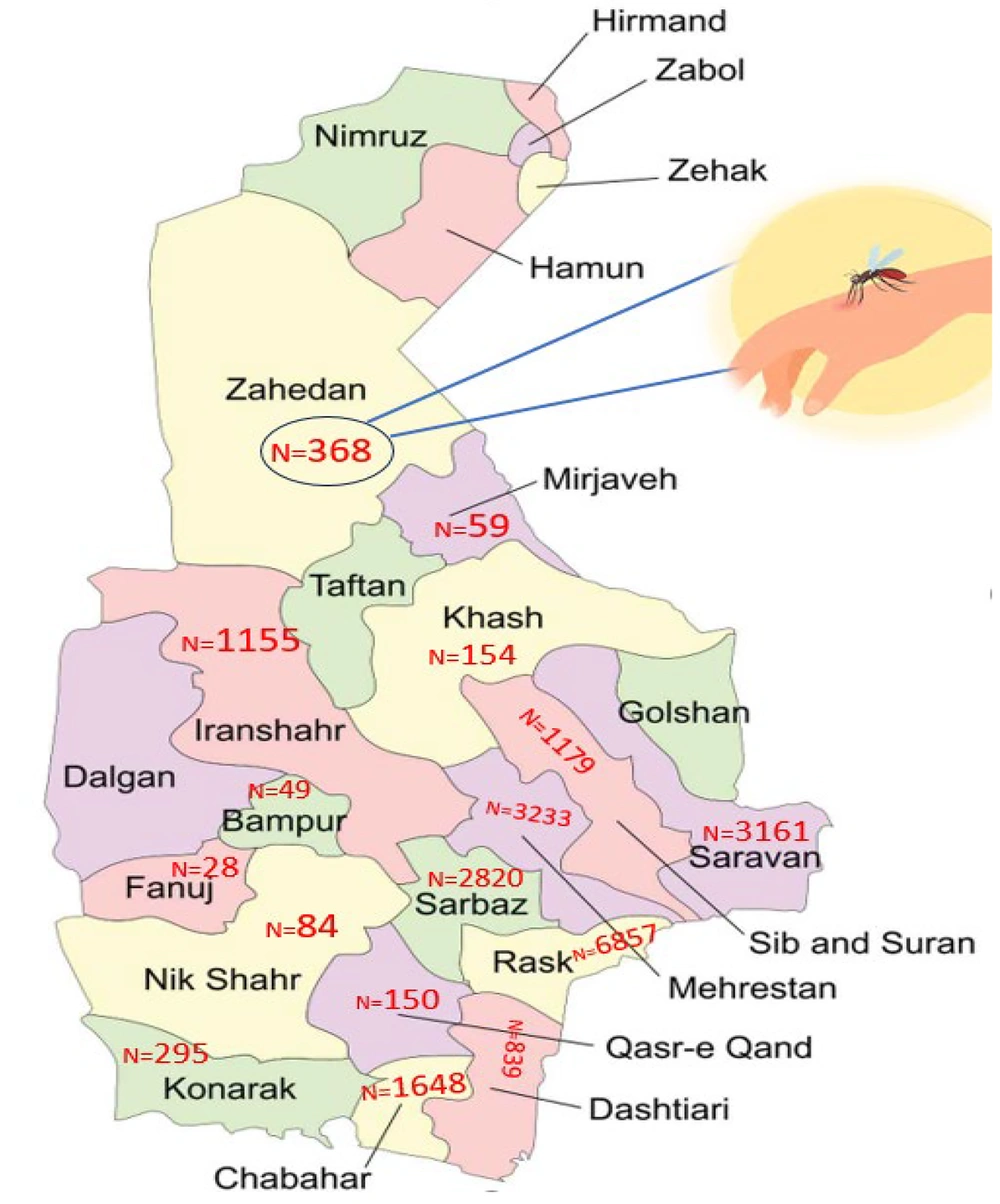
As depicted in Figure 1, the occurrence of malaria cases in the region remained relatively low between 2016 and 2018. However, it began to increase from 2019 onwards and reached its peak in 2023. Additionally, according to Figure 2, the highest incidence of malaria cases was observed in September, coinciding with the summer season in Iran. According to Figure 3, most of the malaria occurred in the border regions with Pakistan (Rask, Mehrestan, and Saravan). Regions that are further away from Pakistan have a lower prevalence of malaria disease. According to Table 2, more men with malaria are diagnosed by the passive care system compared to women. There was a relationship between gender and the type of parasite (P = 0.001). Additionally, an association was observed between the service provider system and the type of care and type of malaria parasite (P = 0.001).
| Variables | Active | Passive | P-Value | Plasmodium vivax | Plasmodium falciparum | Mix Species | P-Value |
|---|---|---|---|---|---|---|---|
| Gender | 0.001 | 0.001 | |||||
| Male | 6208 | 13015 | 13667 | 5220 | 336 | ||
| Female | 1181 | 1675 | 2170 | 652 | 34 | ||
| Service provider university | 0.001 | 0.001 | |||||
| ZAUMS | 1558 | 3363 | 3556 | 1241 | 124 | ||
| IRSHUMS | 5831 | 11327 | 12281 | 4631 | 246 |
Abbreviations: ZAUMS, Zahedan University of Medical Sciences; IRSHUMS, Iranshahr University of Medical Sciences.
5. Discussion
The current study offers a comprehensive overview of the malaria situation during a specific period, encompassing epidemiological data and factors predicting the incidence of this disease, utilizing a large sample size in Iran's Sistan-Baluchistan province. The findings of this study hold significant potential for health decision-makers, aiding in operational planning to control and prepare health facilities for malaria elimination efforts. During the studied period, there has been a general upward trend in the occurrence of malaria in Sistan-Baluchistan province. In 2016, a total of 156 cases were reported, which decreased slightly to 134 cases in 2017.
The implementation of the malaria elimination program in Iran, supported by the WHO since 2009, has played a role in restricting malaria transmission in the southern and southeastern regions of the country (12). This information is supported by previous research, which indicated a decline in malaria incidence in Iran from 0.24 cases per 1000 people in 2002 to 0.01 cases per 1000 people in 2017 (23). Our findings showed a notable rise in the incidence rate of malaria, with the number of infected individuals increasing from 165 cases in 2018 to 852 cases in 2021. Surprisingly, in 2022, there was a sudden and unexpected surge in the disease, resembling an epidemic, resulting in a significant increase to 5034 reported cases, and in 2023, the number of infected people reached 9341.
A study conducted in Iran in 2023 revealed that the number of individuals testing positive for malaria in Sistan-Baluchistan province was about ten times higher in 2022 compared to the previous year, 2021 (2). The rise in malaria cases can be attributed to several factors, including an increase in malaria cases in neighboring countries such as Pakistan, as well as a rise in the number of migrant workers from these countries entering Iran. Additionally, the increase in temperature and heavy monsoon rains may have contributed to the spread of the disease. Improved diagnostic methods in recent years have also led to the identification of new cases (2, 13). Furthermore, the coexistence of COVID-19 and malaria is believed to have played a role in the outbreak of malaria during this period (24). It is crucial for the country's healthcare system to pay closer attention to the effective control of endemic diseases, particularly during the summer season.
The present findings demonstrated that the cities of Rask, Mehrestan, Saravan, and Sarbaz, located near the Pakistan border, have the highest malaria incidence. It was also observed that approximately 65% of the infected individuals traveled to Pakistan during the year of infection. The migration of infected refugees from one malaria-endemic country to another can have various impacts on the spread of the infection. These refugees can transfer parasites to the host country and lead to the spread of infection (14). Therefore, the health issues of immigrants should be considered a priority.
According to the results, the majority of malaria cases during the study period were observed in young and middle-aged individuals. This is consistent with the previous study in the southeast of Iran, which also reported a higher prevalence of the disease in people aged 12 and above (25). This high ratio is attributed to occupational and behavioral factors that lead to greater contact with infectious carriers compared to women (23). In this study, approximately 44.9% of infected individuals were drivers to the border areas of Pakistan.
Studies have shown that climate change has a significant impact on malaria and its vectors. Factors such as temperature, rainfall, relative humidity, intensity, and wind direction play crucial roles in the growth, reproduction, and spread of Anopheles mosquitoes, P. parasites, and malaria transmission (26, 27). In the present study, the highest incidence of the disease was observed in September. Another study conducted in Iran indicated that the average monthly temperature below 30°C in September (specifically 29.2°C) was more favorable for the survival and abundance of adult mosquitoes, thereby facilitating their search for hosts (28). Therefore, it is essential to consider climate change when planning malaria control strategies.
This study supports previous research, with Nili et al. warning that without proper interventions, malaria will continue to pose a health concern in southern Iran (29). Furthermore, a critical factor influencing the transmission dynamics of malaria is the behavioral traits exhibited by Anopheles mosquitoes, particularly their endophilic (indoor resting) and exophagic (outdoor feeding) preferences. Endophilic species generally rest inside human habitation, making them more vulnerable to control measures such as indoor residual spraying (IRS) and the deployment of insecticide-treated nets (ITNs).
Conversely, exophagic mosquitoes primarily obtain nourishment in outdoor settings, thereby constraining the effectiveness of control strategies that are predominantly indoor-focused. These behavioral tendencies highlight the imperative for comprehensive vector control approaches that are customized to the local mosquito ecology, which encompasses specific outdoor interventions, including larval source management and the application of spatial repellents (30).
Our findings showed that P. vivax was identified as the primary and most prevalent cause of malaria among all infected patients. This observation aligns with previous studies conducted in Iran, where this species of parasite was consistently found to be the predominant one. For instance, in studies conducted in Kermanshah and Mazandaran, P. vivax accounted for 98% of all cases (25, 31). Similarly, in the Konarak study, it constituted 91.5% of the cases, while in the Razavi Khorasan study, it comprised 96.4% of the cases (32). Moreover, Rezaei Kahkha-Zhaleh et al., in their study in 2024, found that most malaria cases in Rask County in southeastern Iran were caused by P. vivax, although the prevalence of P. falciparum has been increasing in this area since 2022 (25).
According to the World Health Organization report in 2024, 58 percent of malaria cases in the Eastern Mediterranean region are caused by vivax. Also, according to this report in Iran, although falciparum cases are increasing, most malaria cases in Iran are still related to vivax (3). The results of the present study indicated that nearly 80% of patients received treatment within the first three days of diagnosis. This highlights the importance of conducting epidemiological investigations and maintaining a robust malaria surveillance system capable of effectively detecting active cases, especially asymptomatic cases (33). Additionally, it is crucial to raise awareness among migrant families about the availability of proper malaria prevention services in primary healthcare centers.
Overall, in the malaria elimination program, it is imperative to ensure equitable access to malaria preventive measures for all individuals (34). The result of this study showed that more men with malaria are diagnosed by the passive care system compared to women. Men may be less likely to seek medical care due to various reasons, such as reluctance to express emotions or concerns about their health, feelings of embarrassment, anxiety, fear, and poor communication with healthcare professionals (35), which could contribute to the observed difference in diagnoses between men and women in the passive care system.
5.1. Limitations
Our study had limitations that should be considered. It was based on data sourced from the malaria surveillance system. Therefore, patients for whom the malaria diagnosis checklist was reported were included in the analysis, while patients whose information was not recorded due to lack of access were excluded. Additionally, the present study's findings are specific to only one province with unique conditions, and their generalization to other provinces should be approached with caution. Furthermore, socio-economic, environmental, and cultural factors affecting malaria transmission were not investigated in this study, limiting the depth of understanding of disease dynamics.
5.2. Conclusions
To effectively manage malaria in malaria-prone areas like Sistan-Baluchistan province, health decision-makers are advised to consider the following measures: Promptly treating individuals diagnosed with malaria and implementing vector control strategies, including IRS, long-lasting insecticidal nets, and environmental control measures in the southern areas. It is also important to increase awareness and vigilance regarding the symptoms of malaria, particularly among men, especially those working at the borders and drivers who transport goods across them. The country's healthcare system must pay closer attention to the effective control of endemic diseases, particularly during the summer season. Additionally, the health issues of immigrants should be considered a priority, and it is crucial to raise awareness among migrant families about the availability of proper malaria prevention services in primary healthcare centers. These measures will contribute to the proper management of the disease in these high-risk areas.