1. Background
Acinetobacter baumannii is a Gram-negative, non-fermenting coccobacillus that has emerged as a significant nosocomial pathogen, particularly in intensive care units (ICUs) and burn wards. Its ability to cause severe, hard-to-treat infections and its remarkable capacity to develop multidrug resistance (MDR) has made it a formidable challenge for healthcare systems worldwide. The World Health Organization (WHO) has classified A. baumannii as a "critical priority" pathogen, emphasizing the urgent need for new therapeutic strategies to combat its spread (1). The pathogenicity of A. baumannii is driven by several virulence factors, including biofilm formation and capsular polysaccharide production. Biofilms enable the bacterium to adhere to surfaces, such as medical devices, and protect it from both host immune responses and antibiotics (2). Capsular polysaccharides further enhance its virulence by shielding it from phagocytosis and other immune defenses (3). These traits and its ability to survive in harsh environments make A. baumannii a persistent threat in healthcare settings. Studies have shown that the bacterium can survive on dry surfaces for up to 30 days, with survival prolonged in moist conditions, complicating infection control efforts (4).
One of the most alarming features of A. baumannii is its capacity to develop MDR, especially to carbapenems, which are often regarded as last-resort treatments. The rise of carbapenem-resistant A. baumannii (CRAB) has severely limited therapeutic options, leaving clinicians with few effective antibiotics (5). Resistance to carbapenems is primarily mediated by the production of carbapenem-hydrolyzing class D β-lactamases (CHDLs), such as OXA-23 and OXA-24, which degrade carbapenems and other β-lactam antibiotics (6). Additionally, efflux pump overexpression and alterations in outer membrane permeability contribute to its resistance profile (7). As a result, treatment often relies on polymyxins and combination therapies, which are associated with higher toxicity and limited efficacy (8).
Carbapenem resistance in A. baumannii is primarily driven by carbapenemase enzymes encoded by genes such as blaOXA-23, blaOXA-48, blaNDM, and blaVIM. These enzymes hydrolyze carbapenems, rendering them ineffective. For example, blaOXA-48, frequently found in Enterobacteriaceae, can transfer between bacterial species, spreading resistance (9). Similarly, blaNDM and blaVIM significantly contribute to resistance in Pseudomonas aeruginosa and A. baumannii (10). These genes are often linked to high rates of MDR, complicating treatment and infection control efforts (11).
In the Middle East, including Iraq, the prevalence of CRAB is rising, posing significant challenges to healthcare systems already strained by limited resources and high rates of hospital-acquired infections. Globally, CRAB has been reported with high prevalence in regions such as Europe (e.g., Greece and Italy) and Asia (e.g., China and India), reflecting a widespread challenge to infection control (12, 13). A study from Jordan reported 98.1% resistance to meropenem and 87.7% resistance to imipenem among CRAB isolates, highlighting the severity of the issue (14). In Iran, genomic analyses have revealed the presence of multiple antibiotic resistance genes in CRAB strains, underscoring the ongoing evolution and spread of these pathogens (11). The increasing rates of CRAB infections are particularly concerning in resource-limited settings, where infection control measures and access to effective antibiotics are often inadequate (12).
In Iraq, particularly in the Wasit region, data on the molecular epidemiology of carbapenemase genes in A. baumannii isolates are limited. However, studies in Erbil have identified blaOXA-23 and blaOXA-51 as the most prevalent carbapenemase genes among isolates, with ST 556 and ST 218 being the most common sequence types (15). Another study in Iraq found that all tested isolates carried blaOXA-23 and blaOXA-51, with some also harboring blaOXA-58 (16). These findings highlight a concerning level of carbapenem resistance in Iraq, though data from the Wasit region remain scarce. Understanding the prevalence and distribution of carbapenemase genes is crucial for developing effective infection control strategies and guiding empirical antibiotic therapy. The genetic adaptability of A. baumannii, combined with its ability to thrive in hospital environments and disseminate resistance genes via mobile genetic elements (MGEs), has made CRAB a major public health threat (17). Global travel further facilitates the spread of resistant strains into new environments, underscoring the need for coordinated international efforts to address this growing crisis (18).
2. Objectives
This study aimed to: (1) Detect the presence of carbapenemase genes (blaOXA-23, blaOXA-48, blaNDM, and blaVIM) in A. baumannii isolates from Al-Azizya Hospital, Wasit, Iraq; (2) assess their antibiotic resistance patterns; and (3) analyze the distribution of these genes and resistance profiles to enhance understanding of CRAB’s molecular epidemiology in Iraq and guide local infection control and antibiotic stewardship strategies.
3. Methods
3.1. Sample Collection
Clinical specimens, including urine, wound swabs, blood, and respiratory secretions, were collected from 112 patients (49 in the ICU and 63 in non-ICU wards) at Al-Azizya Hospital, Wasit, Iraq (Figure 1), from May to November 2023, following standard microbiological protocols (19).
3.2. Phenotypic Characterization of the Isolates
Clinical specimens were cultured on MacConkey and blood agar at 37°C for 24 - 48 hours to isolate A. baumannii (19). Suspected colonies, identified by their non-lactose-fermenting, smooth, and mucoid appearance, were subjected to Gram staining to confirm gram-negative coccobacilli morphology. Phenotypic identification was performed using standard biochemical tests, including oxidase (negative), catalase (positive), citrate utilization (positive), motility (non-motile), and growth at 44°C (positive). The API 20NE system (bioMérieux, France) was used to confirm identification, following the manufacturer’s instructions.
3.3. Antibiotic Susceptibility Testing
Antibiotic susceptibility was assessed using the disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI) guidelines (M100, 33rd edition, 2023) (20). The following antibiotics were tested: Ceftazidime, cefotaxime, ciprofloxacin, ceftriaxone, gentamicin, amikacin, aztreonam, imipenem, and meropenem.
3.4. Molecular Detection of Carbapenemase Genes
DNA was extracted from bacterial isolates using a simple boiling method (21). PCR was performed to detect the presence of blaOXA-23, blaOXA-48, blaNDM, and blaVIM genes using specific primers listed in Table 1. The blaOXA-51 gene, intrinsic to A. baumannii, was used as a confirmatory marker for species identification (22). Acinetobacter baumannii (ATCC 19606) was used as the positive control, and molecular-grade water was used as the negative control in all PCR assays. PCR products were visualized on 1.5% agarose gels stained with ethidium bromide and compared against a DNA 100 bp size marker for identification (22).
| Target Genes and Primer Sequence (5'→3') | PCR Product Size (bp) | Reference |
|---|---|---|
| blaOXA-51 | 353 | (23) |
| F: TAATGCTTTGATCGGCCTTG | ||
| R: TGGATTGCACTTCATCTTGG | ||
| blaVIM | 390 | (23) |
| F: GATGGTGTTTGGTCGCATA | ||
| R: CGAATGCGCAGCACCAG | ||
| blaNDM | 621 | (23) |
| F: GGTTTGGCGATCTGGTTTTC | ||
| R: CGGAATGGCTCATCACGATC | ||
| blaOXA-23 | 300 | (23) |
| F: GATGTGTCATAGTATTCGTCG | ||
| R: TCACAACAACTAAAAGCACTG | ||
| blaOXA-48 | 597 | (23) |
| F: AACGGGCGAACCAAGCATTTT | ||
| R: TGAGCACTTCTTTTGTGATGGCT |
4. Results
4.1. Phenotypic and Genotypic Characterization of Strains
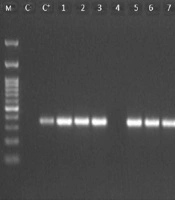
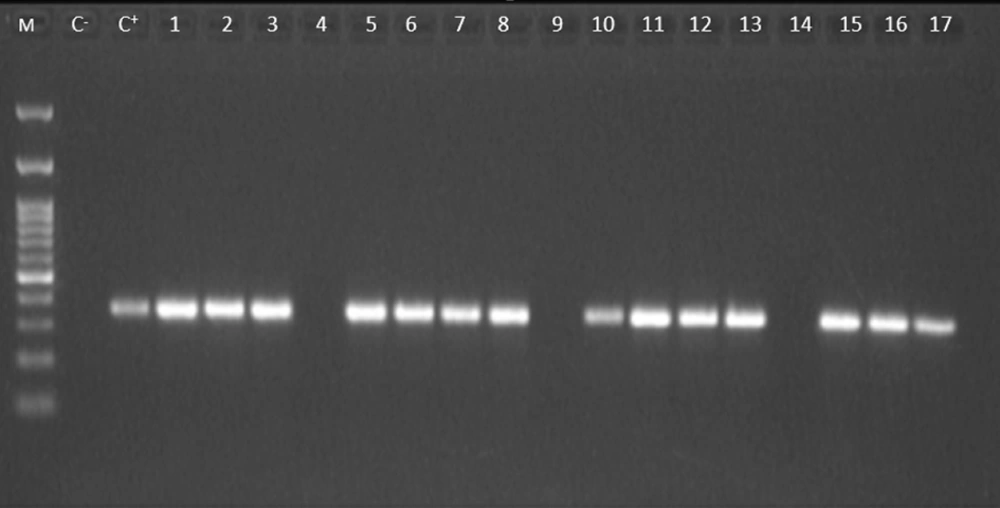
Based on the biochemical and Gram staining results, 63 isolates were identified as A. baumannii. However, using PCR of the species-specific marker blaOXA-51 gene, 58 (92%) isolates were confirmed to be A. baumannii (Figure 2).
4.2. Frequency of Isolates Based on the Type of Patient Wards and Gender
As shown in Table 2, out of 58 isolates, 32 were recovered from 49 patients hospitalized in the ICU, while 26 isolates were collected from 63 patients in the non-ICU wards. Of these, 38 strains (65.5%) were isolated from women, and 20 strains (34.5%) were recovered from men.
| Categories | ICU Wards | Non-ICU Wards | Total |
|---|---|---|---|
| Number of patients | 49 | 63 | 112 |
| Female | 36 | 51 | 87 |
| Male | 13 | 12 | 25 |
| Number of isolates | 32 | 26 | 58 |
| Gender distribution of isolates; No. (%) | |||
| Female | - | - | 38 (65.5) |
| Male | - | - | 20 (34.5) |
4.3. Frequency of Strains Based on the Type of Patient Specimens
As indicated in Table 3, out of 58 A. baumannii strains, the highest number was related to urine samples (43.1%), while the lowest number was related to catheter samples (6.9%).
| Variables | Values |
|---|---|
| Type of specimens | |
| Urine | 25 (43.1) |
| Blood | 14 (24.2) |
| Wound | 9 (15.5) |
| Respiratory secretions | 6 (10.3) |
| Catheter | 4 (6.9) |
| Total | 58 (100) |
a Values are expressed as No. (%).
4.4. Antibacterial Sensitivity Test
Urine samples yielded the highest number of isolates (43.1%), followed by blood (24.2%), wound (15.5%), respiratory secretions (10.3%), and catheters (6.9%).
4.5. Detection of Carbapenemase Genes
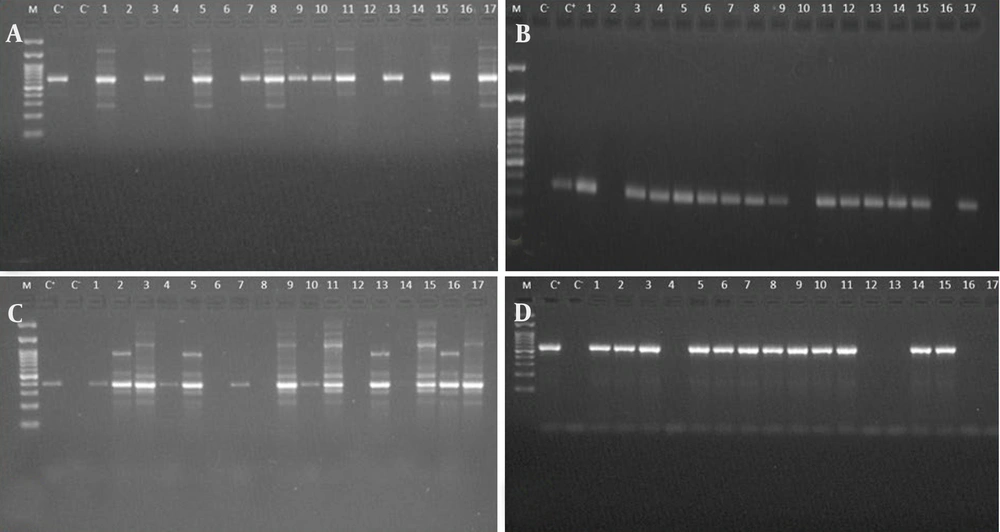
The blaNDM gene was detected in 12 isolates (21%), while the blaVIM gene was found in 14 isolates (24%), and the blaOXA-23 gene was detected in 15 isolates (26%). Additionally, the blaOXA-48 gene was identified in 13 isolates (22%) (Figure 3A - D). The frequency of each gene is summarized in Table 4.
Agarose gel electrophoresis of PCR products for carbapenemase genes detection in Acinetobacter baumannii isolates. A, Lanes: (M) DNA size marker (100 - 1500 bp), (C-) negative control, (C+) positive control, 1 - 17, PCR products from isolates, displaying predictable bands (621 bp), indicative of the amplified blaNDM gene; B, lanes: (M) DNA size marker (100 - 1500 bp), (C-) negative control, (C+) positive control, 1 – 17, PCR products from isolates, exhibiting expected bands (390 bp), representing the amplified blaVIM gene; C, lanes: (M) DNA size marker (100 - 1500 bp), (C+) positive control, (C-) negative control, 1 - 17, PCR products from isolates, displaying expected bands (390 bp), corresponding to the amplified blaOXA-23 gene; D, lanes: (M) DNA size marker (100 - 1500 bp), (C-) negative control, (C+) positive control, 1 - 17, PCR products from isolates, showing a band at 390 bp, representing the amplified blaOXA-48 gene.
| Target Gene | Positive Isolates |
|---|---|
| blaOXA-51 | 58 (100) |
| blaNDM | 12 (21) |
| blaVIM | 14 (24) |
| blaOXA-23 | 15 (26) |
| blaOXA-48 | 13 (22) |
a Values are expressed as No. (%).
5. Discussion
The findings of this study reveal a concerning prevalence of carbapenemase genes among A. baumannii isolates from Al-Azizya Hospital. The blaOXA-51 gene, an intrinsic marker for A. baumannii, was detected in all isolates, confirming their identity. This aligns with global studies reporting the ubiquity of blaOXA-51 in A. baumannii isolates, often accompanied by other carbapenemase genes such as blaOXA-23 and blaOXA-58 (24). The presence of these genes is particularly alarming due to their association with MDR, a significant challenge in healthcare settings (25). The detection of blaOXA-23 (26%), blaVIM (24%), blaOXA-48 (22%), and blaNDM (21%) highlights the genetic diversity of carbapenem resistance mechanisms in this region. These findings are consistent with studies in the Middle East, such as in Iraq and Egypt, where blaOXA-23 was the predominant gene among CRAB and Klebsiella pneumoniae isolates, with blaNDM also contributing significantly to resistance profiles (15, 16, 26). Similarly, a Jordanian study reported high resistance to meropenem (98.1%) and imipenem (87.7%), mirroring our observed rates (96% and 95%, respectively) (14).
However, contrasting results have been reported in other regions. For instance, a study in South Korea found a lower prevalence of blaNDM (5%) among A. baumannii isolates, with blaOXA-23 dominating at 80%, suggesting regional variations in resistance gene distribution (13). In Europe, particularly in Greece, blaVIM was more prevalent than blaOXA-23 in some hospital settings, highlighting differing resistance mechanisms (12, 18, 22). These comparisons underscore the need for region-specific surveillance to tailor infection control strategies.
The presence of blaOXA-23 is particularly concerning due to its association with high-level carbapenem resistance and its role in hospital outbreaks. This gene is often linked to insertion sequences that enhance its expression and mobilization, contributing to the virulence and resistance of A. baumannii (27). Similarly, detecting blaVIM and blaNDM, which encode metallo-β-lactamases, further complicates treatment options. These genes confer resistance to a broad range of β-lactam antibiotics, including carbapenems, which are often considered last-resort treatments for severe infections (28). The emergence of these resistance genes poses a significant public health threat, necessitating alternative treatment strategies such as combination therapies or newer antibiotics like cefiderocol (29).
The antibiotic resistance patterns observed in this study reflect a global trend of increasing resistance among A. baumannii isolates. The highest resistance rates were observed against ceftazidime (100%), meropenem (96%), and imipenem (95%), which are critical antibiotics for treating MDR infections. These findings are consistent with reports from other regions, highlighting the urgent need for enhanced surveillance and infection control measures to mitigate the spread of resistant strains (14). The COVID-19 pandemic has further exacerbated these trends, with shifts in bacterial prevalence and resistance profiles observed during this period (30). The resistance to cephalosporins and carbapenems underscores the need for alternative treatment options and stricter antibiotic stewardship programs. Newer agents such as ceftazidime-avibactam, meropenem-vaborbactam, and cefiderocol have shown promise against resistant strains and should be considered in treatment regimens (31). Effective stewardship programs are essential to optimize antibiotic use, reduce unnecessary prescriptions, and monitor resistance patterns, thereby preserving the efficacy of existing antibiotics (32).
While aztreonam retains some utility against CRAB, with a resistance rate of approximately 59%, its effectiveness varies depending on the specific resistance mechanisms present. Susceptibility testing is therefore crucial before treatment (33). Similarly, the high resistance rates to aminoglycosides (50.2%) and fluoroquinolones (65.7%) among CRAB isolates further limit therapeutic options, necessitating the exploration of alternative therapies and combination treatments (34, 35).
The high prevalence of carbapenemase genes and antibiotic resistance in A. baumannii isolates from Al-Azizya Hospital underscores the urgent need for stringent infection control measures. The ability of A. baumannii to form biofilms enhances its persistence in clinical settings, complicating eradication efforts (36). To mitigate the spread of resistant strains, healthcare facilities must implement rigorous infection control protocols, including enhanced surveillance, strict hand hygiene, contact precautions, and environmental disinfection (37). Continuous education and training for healthcare workers are also vital to ensure compliance with these measures (38).
While this study provides valuable insights into the molecular characteristics of A. baumannii isolates, it is limited by a small sample size from a single hospital and a focus on carbapenemase genes without exploring other resistance mechanisms. Clinical variables such as patient age, underlying diseases, and immunodeficiency status were not examined. Future studies should incorporate these factors to identify risk factors for CRAB infections and inform targeted prevention strategies.
5.1. Conclusions
This study demonstrates the high prevalence of carbapenemase genes and antibiotic resistance in A. baumannii isolates from Al-Azizya Hospital in Wasit, Iraq. The presence of blaOXA-23, blaVIM, blaOXA-48, and blaNDM genes highlights the genetic diversity of carbapenem resistance mechanisms in this region. These findings underscore the urgent need for effective infection control measures, including strict antibiotic stewardship and enhanced surveillance of CRAB in Iraqi hospitals. Additionally, the development of new therapeutic strategies, such as combination therapies and novel antibiotics, is essential to combat the growing threat of multidrug-resistant A. baumannii.