1. Background
Host proteins are utilized by viruses for wide replication and successful release. The rabies virus (RABV) results in to a fatal disease in animals and humans. It belongs to the Lyssavirus genus and the Rhabdoviridae family, characterized by a negative single-stranded RNA with a 12 Kb genome. The genome is covered by nucleocapsid proteins that attach to RNA polymerase and its cofactor P protein (1, 2). The helical structure of the nucleocapsid is related to the matrix protein and enveloped through a membrane containing glycoproteins. Since the virus enters host cells through an acidic membrane fusion process, the polymerase complex carries out viral transcription and replication processes. The RABV P protein plays a vital role in viral transcription and replication. P protein has been identified as an interferon (IFN) antagonist because it prevents IFN induction by disrupting IFN regulatory factor-3 phosphorylation and dimerization, which inhibits IFN production (3, 4).
A yeast two-hybrid screen displayed that RABV P interacts with focal adhesion kinase (FAK) (5). The FAK is a cytoplasmic tyrosine kinase that plays a crucial role in cell signaling pathways (6), which are essential for transcriptional regulation (7), control (8), development (9), and migration (10) of the cell cycle, modification of apoptosis (8, 11), and trafficking of transformed cells (12-14). Some research has shown that the interaction of the viral P protein with FAK positively influences the promotion of rabies infection (5).
Some chaperones vigorously assist with the maturation, stabilization, and activation of cellular or viral kinases/kinase-like targets. As a chaperone, heat shock protein 90 (Hsp90) regulates its structure and activity by binding to adenosine triphosphate (ATP) through its N-terminal binding domain (NBD). Upon binding, NBD transitions to the “closed” state, allowing it to attach to the target protein, facilitating conformational maturation of the protein, and preserving the target in an active state to carry out its function. Then, adenosine triphosphatase (ATPase) splits the ATP into adenosine diphosphate (ADP) and inorganic phosphate (Pi), causing Hsp90 to transition to the “open” state, allowing the target protein to dissociate from Hsp90. Cell division cycle protein 37 (Cdc37/p50), as a co-chaperone to Hsp90, helps regulate protein kinase activity. The Hsp90/cell division cycle 37 (Cdc37) complex controls the folding of certain protein kinases and is central to numerous intracellular signaling networks, thereby regulating a wide range of cellular pathways (15). Both Hsp90 and FAK are proteins that significantly contribute to some disease development, and inhibition of each protein has been shown to diminish tumor progression in animal models (16).
2. Objectives
Previously, research has shown that certain cellular chaperones play a role in RABV infection (17), prompting us to examine the combined impact of these factors on RABV replication. By exploring the disruption of interactions between the P protein and FAK, Hsp90, and Cdc37, we may uncover significant changes in the replication of the RABV P protein and its essential function in RABV infection.
3. Methods
Rabbit polyclonal antibody against rabies P protein was purchased from Abbexa (London, UK). Anti-FAK, anti-Hsp90, anti-Cdc37, anti-β-Actin, goat anti-rabbit IgG, and goat anti-mouse IgG antibodies were obtained from Abcam (USA). The PF-573,228, a FAK inhibitor, was purchased from MedChem Express (USA).
3.1. Cell Culture, Virus Infection, Treatment with an Inhibitor, and MTT Assay
Neuro2a (N2a) cells were cultured in Dulbecco’s modified eagle’s medium (DMEM) supplemented with 5% heat-inactivated fetal calf serum (FCS) (10% v/v) and antibiotics, then incubated at 37°C with 5% carbon dioxide (CO2). Cells were infected with challenge virus standard 11 (CVS-11) [multiplicity of infection (MOI) = 1], and cell lysates were prepared 24 and 48 hours post-infection (hpi) to observe the effect of the virus on the expression of FAK, Hsp90, and Cdc37 proteins. In another experiment, CVS-11 infected cells were treated with various concentrations of PF-573,228 at different time periods before harvesting. Dimethyl sulfoxide (DMSO), as the vehicle for the inhibitors mentioned above, was used as anon-treatment control.
3.2. Plasmid Constructs and Transfection
The murine FAK, Hsp90, and Cdc37 sequences were purchased in commercial vectors from Sino Biologicals Inc. (Germany). The specific primers for constructs generated in this study are listed in Table 1. In order to test multiple genes simultaneously in a virus-infected cell, each gene sequence must be cloned into a specific expression vector. For the construction of recombinant plasmids, the Hsp90 gene was cloned into the plasmid pCI-neo (Promega, USA), the Cdc37 gene was cloned into pCMV-script (Clontech, Palo Alto, USA), and the FAK gene was cloned into pcDNA3.1 (Invitrogen, Thermo Fisher Scientific, USA).
| Genes | Vectors | Primers |
|---|---|---|
| FAK | pcDNA3.1 | F: 5' AAG GGA TCC ACC ATG GCA GCT GCT TAT CTT G 3' |
| R: 5' GTG GTG GTG GTG GTG TGG TCG TGT CTG CCC TAG 3' | ||
| Hsp90 | pCI-neo | F: 5' AAG CTA GCC ACC ATG GTG CCT GAG GAA ACC C 3' |
| R: 5' GTG GTG GTG GTG GTG ATG GTC TAC TTC TTC C 3' | ||
| Cdc37 | pCMV-Script | F: 5' AT GAA TTC GCC ACC ATG GTA GAC TAC AGC 3' |
| R: 5' GTG GTG GTG GTG GTG CGC ACT GAC GTC TTT C 3' |
List of Primers Designed for Focal Adhesion Kinase, Heat Shock Protein 90, and Cell Division Cycle 37
3.3. Western Blot Analysis
A Western blot analysis was conducted on normal and infected cells to compare the levels of FAK, Hsp90, Cdc37, and P proteins. The cell lysate was prepared by adding lysis buffer [50 mM Tris-hydrochloride (Tris-HCl) pH 8, 150 mM sodium chloride (NaCl), 0.1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride (PMSF)] to samples on ice for 5 minutes. Samples were re-suspended in 4 × sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and boiled for 8 minutes. After centrifugation, the soluble cell lysates were separated on 10 - 12% SDS-PAGE gels. Subsequently, the gels were transferred to polyvinylidene fluoride (PVDF) membranes for an overnight blotting process at 4°C. Membranes were then blocked with 5% skim milk in PBS for 1 hour at 37°C and incubated with the specific primary antibodies overnight at 4°C. After washing, the membranes were incubated with the secondary antibody conjugated to horseradish peroxidase (HRP) for 1 hour at 37°C. The blots were exposed to a 3,3′-diaminobenzidine-tetrahydrochloride (DAB) substrate kit (34002, Thermo Fisher Scientific, USA).
3.4. Short Hairpin RNA Constructs and Transfection
The Hsp90 or Cdc37 knockdown was conducted in N2a cells using the vector-based shRNA approach with pCDH-EF1-copGFP and pEGFP-C1 vectors. The shRNA targeting sequences were designed using two online design algorithms: Selection program siRNA and siRNA Wizard Software. Sense and antisense primers were designed for Cdc37 (S 5'-AAT TCG GAT GAT CAG ATG CTG CAA GAC GAA TCT TGC AGC ATC TGT ACA TCC-3', AS 5'-GAT CCG GAT GTA CAG ATG CTG CAA GAT TCG TCT TGC AGC ATC TGT ACA TCC-3') and Hsp90 (S 5'-AAT TCG GAA GGA GCT GCA CAT CAA TCC GAA GAT TGA TGT GCA GCT CCT TCC-3', AS 5'-GAT CCG GAA GGA GCT GCA CAT CAA TCT TCG GAT TGA TGT GCA GCT CCT TCC-3') with two restriction enzyme sites for BamHI and EcoRI. The N2a cells at 80% confluency were transfected with shRNA constructs using Lipofectamine 3000 transfection reagent (L3000001, Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. After 24 hours of growth at 37°C, cells were infected with CVS-11 (MOI = 1) for the specified durations.
3.5. Active Inhibition of FAK After RABV Infection and Its Effect on Rabies P Protein, Hsp90, and Cdc37
The N2a cells were cultured in DMEM supplemented with FCS (10% v/v) and incubated at 37°C with 5% CO2 overnight. Then, the cells were transfected with all three vectors via Lipofectamine 3000 (Thermo Fisher). Cells were then infected with CVS-11 RABV (MOI = 1). To ensure cloning accuracy, total protein was extracted from N2a cells via lysis buffer at 24 and 48 hpi. Western blot analysis was used to confirm the expression of FAK, Hsp90, Cdc37, and viral P protein. β-actin was used as a loading control in Western blot analysis to normalize the levels of detected protein by ensuring consistent protein loading throughout the gel.
3.6. Hsp90 and Cdc37 Knockdown and RABV Infection
The N2a cells were cultured in DMEM supplemented with FCS (10% v/v) and incubated at 37°C with 5% CO2 overnight. Then, the cells were transfected with a scramble vector for 24 hours via Lipofectamine 3000 and infected with CVS-11 (MOI = 1) for 48 hpi. In the second experiment, we transfected cells with pEGFP-C1-ShHsp90 for 24 hours. Cells were then infected with CVS-11 for 48 hpi. In the third experiment, the cells from the second experiment were exposed to 1 μM PF-573,228 for 24 hours. In this way, we could investigate the effect of inhibiting FAK and knocking down Hsp90 simultaneously on the rabies P protein expression. Cell lysates from the three groups were collected to investigate Hsp90 expression by Western blot analysis. We followed the same procedure with the pCDH-CMV-MCS-ShCdc37 vector for Cdc37.
3.7. Virus Titration
The supernatant from virus-infected cells was collected in its normal state, overexpressed, and inhibited by drug or gene knockdown to conduct a plaque assay on days 0, 2, 5, and 10. The plaque assay was carried out as described by Webber et al. (16).
3.8. Statistical Methods
The normal distribution of all quantitative variables was assessed using the Shapiro-Wilk test. The gene expression levels between two groups were analyzed using the Student t-test, while more than two groups were analyzed using one-way ANOVA with Dunnett’s multiple comparisons test. If the distribution was not normal, nonparametric alternative tests were used. Analysis and graph plotting were conducted using GraphPad Prism version 9.0.2.
4. Results
4.1. MTT Assay, Evaluation of FAK, Hsp90, and Cdc37 in Virus-Infected Cells
We performed an MTT assay to determine the appropriate concentration of PF-573,228, which is not only non-toxic for N2a cells but also has an inhibitory effect on FAK protein expression. We found that a concentration of 1 μM caused the least amount of cell death in N2a cells after 48 hours (Figure 1A). To determine the effect of drug concentration on the expression of RABV P protein, we conducted a Western blot analysis and found the lowest protein expression at a 1 μM (1000 nM) concentration (Figure 1B). The N2a cells were then infected with CVS-11 (MOI = 1) for two hours, and the cell lysates were examined for the presence of FAK, Hsp90, and Cdc37 proteins at 24 and 48 hpi. As the rabies infection progressed in the cells, the expression of these three proteins increased (Figure 2A). When a quantitative analysis, such as a Student t-test, was carried out on the levels of FAK, Hsp90, and Cdc37, significant changes were observed (Figure 2B). GraphPad Prism version 9.0.2 was used to draw graphs. The expression of FAK, Hsp90, and Cdc37 genes in virus-infected nerve cells increased at 24 hpi, reached its maximum at 48 hpi, but infection did not affect the expression of β-actin as a cell control (Figure 2B).
A, MTT assay performance to determine the proper concentration of PF-573,228. In concentration of 1 μM of drug we observe the least amount of death in neuro2a (N2a) cells after 48 hours. Therefore, in our experiments, we always used this effective and non-toxic concentration for cells; B, the effect of different concentrations of PF-573-228 on the P protein of CVS-11. The N2a cells were grown until reaching appropriate confluency, then they were trypsinized, and finally, the challenge virus standard-11 (CVS-11) [multiplicity of infection (MOI) = 1] was added. Two hours later, PF-573-228 was added at concentrations of 0, 0.25, 0.5, and 1 μM. As is known, at concentrations greater than 1 μM, the expression of the P protein decreases and reaches its lowest level at 1 μM. However, it does not stop entirely because the drug only inhibits focal adhesion kinase (FAK). Low levels of P protein expression are due to the additional influence of other chaperones.
Neuro2a (N2a) cells were infected with the challenge virus standard-11 (CVS-11) for 2 hours [multiplicity of infection (MOI) = 1], and then the cell culture medium was changed. A, cell lysates were collected at 24 and 48 hours post-infection (hpi). Immunoblotting analysis was performed using rabbit monoclonal antibodies against focal adhesion kinase (FAK), cell division cycle 37 (Cdc37), β-actin, and a mouse monoclonal antibody against heat shock protein 90 (Hsp90); B, quantitative analysis of cellular proteins (FAK, Cdc37, Hsp90, and β-actin). Student t-test analysis was conducted to compare the quantitative expression of three proteins (FAK, Hsp90, and Cdc37) with β-actin at 24 and 48 hpi in rabies virus (RABV)-infected cells. The expression of all three proteins shows a significant increase, with the maximum expression is observed at 48 hpi. The infection does not affect the β-actin expression, which serves as a control (**** P < 0.0001).
4.2. Active Inhibition of FAK Using PF-573,228
To investigate the active inhibition of FAK, N2a cells were infected with CVS-11 and exposed to PF-573,228 (1 μM) 2 hours later. Western blot analysis showed no bands for FAK at 24 and 48 hpi because PF-573,228 is a strong kinase inhibitor, while it did not affect the expressions of Hsp90, Cdc37, and β-actin (Figure 3A). Additionally, we performed a quantitative analysis of FAK, Cdc37, Hsp90, and β-actin in cells infected with CVS-11 and exposed to PF-573,228 (1 μM) at 24 and 48 hpi. PF-573,228, as a kinase inhibitor, blocked FAK at 24 and 48 hours but did not affect Hsp90, Cdc37, or β-actin (Figure 3B).
A, Western blot analysis of neuro2a (N2a) cells. In lane 1, normal N2a cells were infected with challenge virus standard-11 (CVS-11) [multiplicity of infection (MOI) = 1] and exposed to 1 μM PF-573,228 for 24 hours. Lane 2 showed the same cells 48 hours post-infection (hpi). Focal adhesion kinase (FAK) expression was completely inhibited by PF-573,228, a FAK inhibitor. However, no changes were observed in the expression heat shock protein 90 (Hsp90) and cell division cycle 37 (Cdc37). β-actin is considered a control protein; B, quantitative analysis of FAK, Cdc37, Hsp90, and β-actin in cells infected with CVS-11 and exposed to PF-573,228 (1 μM) at 24 and 48 hpi. The Hsp90 and Cdc37 expressions were compared with β-actin expression. Significant changes were observed in Hsp90 at 24 hpi, while changes occurred for Cdc37 at 48 hpi (* P < 0.05). PF-573,228, a kinase inhibitor, blocked FAK at 24 and 48 hpi, but it does not affect Hsp90, Cdc37, or β-actin. The higher expression of these two proteins at 48 hpi compared to 24 hpi was due to the prolonged presence of the rabies virus (RABV) infection.
4.3. Investigating the Effects of FAK, Hsp90, and Cdc37 Overexpression on Viral P Protein in CVS-11 Infected Cells
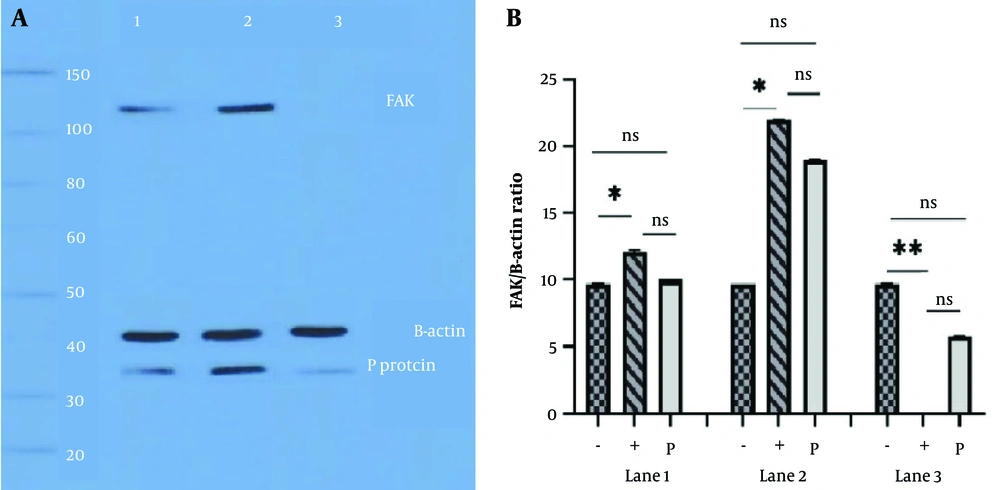
The N2a cells were transfected with expression vectors and then infected with RABV (MOI = 1) to study the effect of FAK, Hsp90, and Cdc37 gene overexpression on viral P protein. Ultimately, these cells were exposed to PF-573,228 to assess its inhibitory effect on the expression of P protein, FAK, Hsp90, Cdc37, and β-actin. When N2a cells were transfected with a pcDNA empty vector, the transfection did not affect the FAK and P protein contents of the cells. After pcDNA-FAK transfection, an increase in FAK and P protein expression was observed, but it had no effect on the control (Figure 4A, lane 2). Upon post-drug administration into these cells, the expression of the P protein was affected by FAK inhibition (Figure 4A, lane 3). Quantitative analysis of overexpression of FAK and β-actin in virus-infected and transfected cells showed a significant increase in FAK expression 48 hours after transfection (Figure 4B, lane 2). The FAK expression was also inhibited in transfected cells exposed to the 1 μM drug, and P protein expression was decreased significantly (Figure 4B, lane 3).
A, focal adhesion kinase (FAK) overexpression in challenge virus standard-11 (CVS-11) infected cells; lane 1, neuro2a (N2a) cells transfected with pcDNA empty vector before infection. The FAK and P protein expressions in the cell lysate were normal. Lane 2, cell transfection with pcDNA-FAK was performed, the virus was inoculated, and cell lysate blotting showed distinct bands for FAK and P protein 48 hours post-infection (hpi). Lane 3, cells in lane 2 were exposed to PF-573,228 (1 μM) two hours after virus inoculation. Cell lysate was applied to Western blot analysis 48 hpi. The lack of an FAK band is due to the inhibitory effect of PF-573,228. The low expression of the viral P protein is due to the additional influences of heat shock protein 90 (Hsp90) and cell division cycle 37 (Cdc37) chaperones. β-actin is considered a control protein; B, quantitative analysis of FAK overexpression and β-actin, in cells infected with CVS-11 and exposed to PF-573,228 (1 μM) at 48 hpi; lane 1, N2a cells were transfected with a pcDNA empty vector before infection. The (+) sign indicates the presence of FAK and the (-) sign indicates the absence of FAK, and only β-actin is present as a control. The FAK and rabies P protein expression in cell lysate were studied, and a significant difference was observed only between β-actin and FAK expression at 24 hpi (* P < 0.05). Lane 2, cell transfection with the pcDNA-FAK vector was performed before virus inoculation. Western blot analysis showed the overexpression of FAK and P protein 48 hpi. We observed significant differences in the expression of β-actin and FAK at 48 hpi (* P < 0.05). Lane 3, N2a cells were exposed to PF-573,228 (1 μM) two hours after virus inoculation, and cell lysates were detected at 48 hpi. Lack of FAK expression, due to the inhibitory effect of PF-573,228, caused significant differences (** P < 0.01). β-actin is considered a control protein.
When we noted that the pCI-neo empty vector had no effect on the Hsp90 and P protein contents of the infected cells, we decided to transfect the infected cells with the pCI-neo-Hsp90 vector for Western blot analysis. Subsequently, we observed a stronger bond between Hsp90 and the viral P protein (Figure 5A, lane 2). Cells were then exposed to PF-573,228 (1 μM) two hours after virus inoculation, which had no effects on Hsp90 and β-actin expressions but did affect the expression of the P protein (Figure 5A, lane 3).
A, heat shock protein 90 (Hsp90) overexpression challenge virus standard-11 (CVS-11) infected cells; lane 1, neuro2a (N2a) cells were transfected with the pCI-neo empty vector before infection. Visible bands correspond to the expression of Hsp90 and rabies P protein in cell lysates at 24 hours post-infection (hpi). Lane 2, cells were transfected with pCI-neo-Hsp90, and cell lysate blotting analysis showed strong bands for Hsp90 and P protein at 48 hpi. Lane 3, N2a cells were inoculated with the rabies virus (RABV), exposed to PF-573,228 (1 μM) two hours later, and checked at 48 hpi. It had no effect on Hsp90 expression. The low expression of the P protein is also due to the additional effect of Hsp90 and cell division cycle 37 (Cdc37) chaperones, as the inhibitor blocks focal adhesion kinase (FAK). β-actin is considered a control protein; B, quantitative analysis of Hsp90 overexpression and β-actin, in cells infected with CVS-11 and exposed to PF-573,228 (1 μM) at 48 hpi; lane 1, normal N2a cells were transfected with a pCI-neo empty vector before RABV infection. Western blot analysis of cell lysate at 24 hpi showed significant differences between β-actin and Hsp90. The (+) sign indicates the presence of Hsp90 and the (-) sign indicates the absence of Hsp90 (** P < 0.01). Lane 2, cells were transfected with pCI-neo-Hsp90, and cell lysate blot analysis revealed significant differences between β-actin and Hsp90 (** P < 0.01) at 48 hpi. Lane 3, N2a cells were infected with the RABV, exposed to PF-573,228 (1 μM) for two hours, and checked at 48 hpi. Although it had no effect on the P protein expression, it caused a significant difference between β-actin and Hsp90 (** P < 0.01). The low expression of the P protein is also due to the accessory effect of Hsp90 and Cdc37 chaperones, as the inhibitor blocked FAK. β-actin is considered a control protein.
In lane 1, cells were transfected with a pCI-neo empty vector and inoculated with the virus. Significant differences were observed only between the presence (+) and absence (-) of Hsp90 (P < 0.01) (Figure 5B, lane 1). Additionally, when the full vector was used, a significant difference was again seen in these two parameters (P < 0.01) (Figure 5B, lane 2). Since the drug had no inhibitory effect on Hsp90 and the control, a statistically significant difference was recorded between Hsp90 and the control (P < 0.01) (Figure 5B, lane 3). It appears that Hsp90 alone cannot exert substantial effects on the expression of the RABV P protein.
In another experiment, we transfected both the pCMV-Script and pCMV-Script-Cdc37 vectors into virus-infected cells. Checking the Cdc37 and P protein expressions by Western blot did not display any changes for the empty vector (Figure 6A, lane 1); however, analysis of the other vector showed an increase in the expression of Cdc37 and RABV P protein (Figure 6A, lane 2), while the control expression did not show any change. Only P protein expression decreased when the infected cells were exposed to the drug (Figure 6A, lane 3). Figure 6B shows the results of quantitative analysis of these experiments for Cdc37 in the pCMV-Script empty vector (Figure 6B, lane 1), overexpression with pCMV-Script-Cdc37 (Figure 6B, lane 2), and treatment with the drug inhibitor. There was a significant difference in pCMV-Script-Cdc37 (P < 0.01) infected cells compared with control cells after 48 hours. Then infected cells were exposed to PF-573,228, but the result showed that the drug had no effect on Cdc37 (Figure 6B, lane 3). The low expression of the P protein is also due to the additional influence of Hsp90 and Cdc37 chaperones as the inhibitor blocked FAK (P < 0.01). It seems that Cdc37 alone cannot cause significant changes in the expression of the RABV P protein.
A, cell division cycle 37 (Cdc37) overexpression in challenge virus standard-11 (CVS-11) infected cells; lane 1, neuro2a (N2a) cells were transfected with a pCMV-Script empty vector and then infected with the rabies virus (RABV) [multiplicity of infection (MOI) = 1] for 24 hours. P protein and Cdc37 expression levels were observed to be normal. Lane 2, cells were transfected with pCMV-Script-Cdc37 and then inoculated with the virus. Western blot analysis of the cell lysate displayed distinct bands of Cdc37 and P protein 48 hours post-infection (hpi). The high expression of the P protein was due to the overexpression of Cdc37. Lane 3, N2a cells were treated with PF-573,228 (1 μM) two hours after virus inoculation and detected 48 hpi. The inhibitor did not affect the expression of Cdc37 protein. Due to the inhibitor of focal adhesion kinase (FAK), a decrease in the expression of the P protein was observed, facilitated by heat shock protein 90 (Hsp90) and Cdc37 chaperones; B, β-actin is considered a control protein. Quantitative analysis of Cdc37 overexpression and β-actin, in cells infected with CVS-11 and exposed to PF-573,228 (1 μM) at 48 hpi. Lane 1, normal N2a cells were transfected with the pCMV-Script empty vector before RABV infection. Western blot analysis of cell lysate at 24 hpi showed significant differences between β-actin and Cdc37. The + sign indicates the presence and the - sign indicates the absence of Cdc37 (** P < 0.01). Lane 2, cells were transfected with pCMV-Script-Cdc37, and cell lysate blot analysis showed significant differences between β-actin and Cdc37 (** P < 0.01) 48 hpi. Lane 3, N2a cells were infected with the RABV, exposed to PF-573,228 (1 μM) for two hours, and checked 48 hpi. Although it did not affect the expression of the P protein, it caused a significant difference between β-actin and Cdc37 (** P < 0.01). β-actin is considered a control protein.
4.4. Hsp90 and Cdc37 Knockdown and RABV Infection
We sought to investigate the effect of Hsp90 and Cdc37 knockdown on infected cells, as well as on infected cells treated with drugs. We transfected N2a cells with a scramble vector and then infected them with the virus. The Hsp90 and P protein levels remained unchanged in these cells (Figure 7, lane 1). We transfected cells with pEGFP-C1-ShHsp90 for 24 hours and then infected the cells with CVS-11 for 48 hpi. Cell lysates were collected and used for Western blot analysis. Results showed that Hsp90 disappeared and the P protein faded (Figure 7, lane 2). In the following step, cells were transfected with pEGFP-C1-ShHsp90 for 24 hours, infected with the virus for two hours, and then treated with 1 μM PF-573,228 for 48 hpi. The fading band of the P protein was due to the additional influence of the chaperone Cdc37, as the inhibitor blocked FAK, and Hsp90 was knocked down (Figure 7, lane 3). β-actin is considered a control protein. This was the outcome when we used pCDH-CMV-MCS-ShCdc37 to knock down Cdc37 (Figure 8, lane 2). We could not see the Cdc37 band because the gene had been knocked down, and FAK was inhibited by the drug (Figure 8, lane 3). The faint band of viral P protein was present only due to the additional influence of Hsp90.
Heat shock protein 90 (Hsp90) knockdown and rabies infection; lane 1, neuro2a (N2a) cells were transfected with a scramble vector and infected with rabies virus (RABV) [multiplicity of infection (MOI) = 1]. Western blot analysis of cell lysate at 24 hours post-infection (hpi) showed that the expressions of Hsp90 and P protein were normal. Lane 2, the lack of the Hsp90 band was due to cell transfection with pEGFP-C1-ShHsp90 for 24 hours followed by infection with challenge virus standard-11 (CVS-11) for 48 hpi. The low expression of the P protein is due to the normal expression of focal adhesion kinase (FAK) and cell division cycle 37 (Cdc37). Lane 3, cells were transfected with pEGFP-C1-ShHsp90 for 24 hours, infected with the virus for two hours, treated with PF-573,228 (1 μM) for two hours, and then checked at 48 hpi. The very low expression of the P protein is also due to the additional influence of the Cdc37, as the inhibitor blocked FAK, and Hsp90 was knocked down by shRNA. β-actin is considered a control protein.
Cell division cycle 37 (Cdc37) knockdown and rabies infection; lane 1, cells were transfected with a scramble vector and infected with challenge virus standard-11 (CVS-11) [multiplicity of infection (MOI) = 1], and the expressions of Cdc37 and P protein were normal. Lane 2, the lack of a Cdc37 band was due to cell transfection with pCDH-CMV-MCS-ShCdc37 for 24 hours and infection with the virus for 48 hours post-infection (hpi). The expression of the P protein is due to the normal expression of focal adhesion kinase (FAK) and Hsp 90. Lane 3, cells were transfected with pCDH-CMV-MCS-ShCdc37 for 24 hours, infected with the virus for two hours, and then treated with 1 μM PF-573,228. The low expression of P protein is also due to the additional influence of heat shock protein 90 (Hsp90). This was caused by the inhibition of FAK and the knockdown of Cdc37. β-actin is considered a control protein.
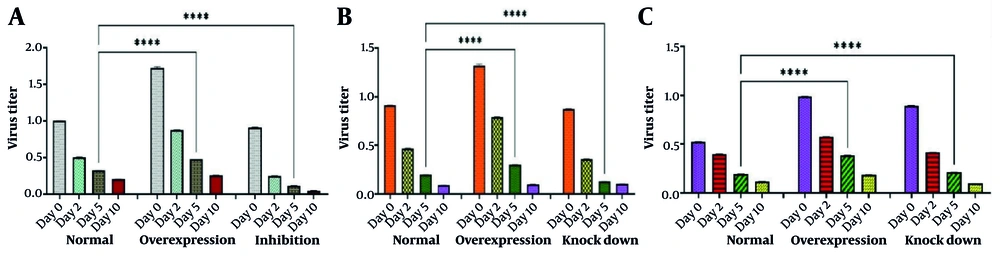
4.5. Virus Titration Results
We estimated the virus titer using a plaque assay on days 0, 2, 5, and 10 when FAK expression was normal, overexpressed, and inhibited by PF-573,228. As shown in Figure 9A, a significant difference was observed on day 5 between the normal and FAK overexpression state (P < 0.0001). The same significant difference was observed when comparing the normal state with PF-573,228-inhibited FAK (P < 0.0001). Differences were observed on other days, but they were not significant. When we studied virus titer in normal, overexpressed, and knocked down states for Hsp90 (Figure 9B) and Cdc37 (Figure 9C), we obtained almost identical results. Significant changes were observed in each condition only on the fifth day. The P-value for significant differences was 0.0001 (P < 0.0001). When we studied virus titer in normal, overexpressed, and knocked down states for Hsp90 and Cdc37, we obtained almost identical results. Significant changes in each condition were observed only on the fifth day.
A, titration of rabies virus (RABV) under normal conditions, focal adhesion kinase (FAK) overexpression, and FAK inhibition by an inhibitor. Neuro2a (N2a) cells were transfected with challenge virus standard-11 (CVS-11). In the normal state, supernatants were collected on days 0, 2, 5, and 10 for virus titration using a plaque assay. When FAK was overexpressed by pcDNA-FAK vector transfection or inhibited by PF-573,228 (1 μM), supernatants were collected in the same way, and plaque assays were conducted. Although the changes in virus titer were higher in the FAK overexpression and lower in the FAK inhibition state compared to normal FAK, significant differences were only observed on the fifth day (**** P < 0.0001); B, titration of RABV under normal conditions, heat shock protein 90 (Hsp90) overexpression, and Hsp90 gene knockdown by pEGFP-C1-ShHsp90. The N2a cells were transfected with CVS-11. In the normal state, supernatants were collected on days 0, 2, 5, and 10 for virus titration using a plaque assay. When Hsp90 was overexpressed by pCI-neo–Hsp90 vector transfection or knocked down by pEGFP-C1-ShHsp90, supernatants were collected in the same way, and plaque assays were carried out. Changes in virus titer were higher in the Hsp90 overexpression state compared to the virus titer in the normal state and the Hsp90 knockdown. This was logical because the role of Hsp90 in virus amplification is second only to FAK. Significant differences were only observed on the fifth day (**** P < 0.0001); C, titration of RABV under normal conditions, cell division cycle 37 (Cdc37) overexpression, and Cdc37 gene knocking down by pCDH-CMV-MCS-ShCdc37. The N2a cells were transfected with CVS-11. In normal state, supernatants were collected on days 0, 2, 5, and 10 for virus titration using a plaque assay. When Cdc37 was overexpressed by pCMV-Script-Cdc37 vector transfection or knocked down by pCDH-CMV-MCS-ShCdc37, supernatants were collected in the same way, and plaque assays were carried out. Changes in the virus titer were higher in the Cdc37 overexpression and Cdc37 knockdown states compared to the normal state. This was logical because the role of Cdc37 in virus amplification is cooperative concerning Hsp90 and is of third-degree importance to FAK. Significant differences were observed only on the fifth day (**** P < 0.0001).
5. Discussion
Inhibition of FAK in cancer cells disrupts the integrin-dependent signaling pathway and stops cell growth (17-19). The relationship between the RABV P protein and FAK has been investigated in some scientific sources (5). Therefore, inhibiting FAK may reduce its interaction with the P protein in RABV-infected cells, potentially reducing virus replication. It is also mentioned that the rabies P protein function is related to the functions of certain chaperones (20-23). Since the RABV P protein plays a critical role in viral RNA synthesis and transcription, reducing the association of Hsp90 and Cdc37 chaperones with it may make the immune system more accessible to the P protein (24, 25). However, the simultaneous relationship of all three with the RABV P protein inside the cell has never been investigated.
All N2a cells have basal levels of Hsp90, FAK, and Cdc37. After infecting these cells with CVS-11, the expression of FAK, Cdc37, and Hsp90 starts increasing at 24 hpi and reaches a maximum at 48 hpi. Only treatment with PF-573,228 at 1 μM (1000 nM) could inhibit the expression of FAK. We also studied the changes in virus titer when exogenous FAK, Hsp90, and Cdc37 were transfected into infected cells compared to normal cells. A significant increase in the amount of P protein and virus titer was observed, indicating a direct relationship between the increase of FAK, Hsp90, and Cdc37 with this protein. Conducting a plaque assay on the supernatant collected from the transfected infected cells also revealed a significant increase in the virus titer on the fifth day compared to the normal condition.
To test this hypothesis, we first inhibited FAK by PF-573,228 in infected cells. We observed the elimination effect on FAK when there were no decreases in Hsp90 and Cdc37, or in the control group. By conducting a Western blot, we found that the P protein band diminished but was not omitted due to additional influences of Hsp90 and Cdc37. A significant decrease in virus titers was observed by conducting a plaque assay on the supernatant of cells treated with the FAK inhibitor, which also showed the same result. By knocking down the Hsp90 and Cdc37 genes in CVS-11 infected cells, a decrease was observed in the P protein, while there was no change in FAK; of course, the virus titer also decreased significantly on the fifth day. When gene inhibition and knockdown were applied to infected cells, they showed an absence of either FAK-Hsp90 or FAK-Cdc37. The P protein band faded, and the virus titer greatly reduced, although it was not completely eliminated due to the additional influence of one of the chaperones.
Some researchers believe that the protein-protein interaction of Cdc37 and Hsp90 with the P protein is involved in the stability, development, and amplification of RABV in cells of neural origin. We could confirm the validity of this hypothesis by conducting our experiments. It should be noted that the role of Cdc37 is auxiliary and independent from the main chaperone role of Hsp90. The FAK is at the intersection of several important cell signaling pathways that can be used by RABV for further replication because the plaque assay revealed that the inhibition of FAK significantly altered virus titer, suggesting that the role of FAK is major. It should be noted that the mechanism by which FAK, Cdc37, and Hsp90 maintain the stability of the viral P protein needs further investigation because there are certainly further factors involved in it.
This research focused on examining how PF-573,228 inhibits FAK and how silencing the Hsp90 and Cdc37 genes impacts the expression of the RABV P protein, which is essential for virus replication. Our biochemical and genetic studies demonstrated that inhibiting FAK and silencing the Hsp90 and Cdc37 genes resulted in lowered expression of the P protein and subsequently reduced the virus titer. Our research revealed that using both approaches together did not inhibit the expression of P protein or the production of RABV, suggesting that RABV utilizes multiple alternative pathways at once to evade the host’s immune response and maintain its replication. These results highlight the importance of conducting more studies to explore the alternative pathways that RABV leverages within host cells to enhance its replication.

![A, MTT assay performance to determine the proper concentration of PF-573,228. In concentration of 1 μM of drug we observe the least amount of death in neuro2a (N2a) cells after 48 hours. Therefore, in our experiments, we always used this effective and non-toxic concentration for cells; B, the effect of different concentrations of PF-573-228 on the P protein of CVS-11. The N2a cells were grown until reaching appropriate confluency, then they were trypsinized, and finally, the challenge virus standard-11 (CVS-11) [multiplicity of infection (MOI) = 1] was added. Two hours later, PF-573-228 was added at concentrations of 0, 0.25, 0.5, and 1 μM. As is known, at concentrations greater than 1 μM, the expression of the P protein decreases and reaches its lowest level at 1 μM. However, it does not stop entirely because the drug only inhibits focal adhesion kinase (FAK). Low levels of P protein expression are due to the additional influence of other chaperones. A, MTT assay performance to determine the proper concentration of PF-573,228. In concentration of 1 μM of drug we observe the least amount of death in neuro2a (N2a) cells after 48 hours. Therefore, in our experiments, we always used this effective and non-toxic concentration for cells; B, the effect of different concentrations of PF-573-228 on the P protein of CVS-11. The N2a cells were grown until reaching appropriate confluency, then they were trypsinized, and finally, the challenge virus standard-11 (CVS-11) [multiplicity of infection (MOI) = 1] was added. Two hours later, PF-573-228 was added at concentrations of 0, 0.25, 0.5, and 1 μM. As is known, at concentrations greater than 1 μM, the expression of the P protein decreases and reaches its lowest level at 1 μM. However, it does not stop entirely because the drug only inhibits focal adhesion kinase (FAK). Low levels of P protein expression are due to the additional influence of other chaperones.](https://services.brieflands.com/cdn/serve/3170b/6b494d7c48258c78fb582db362b2dec318026ed2/jjm-160281-i001-F1-preview.webp)
![Neuro2a (N2a) cells were infected with the challenge virus standard-11 (CVS-11) for 2 hours [multiplicity of infection (MOI) = 1], and then the cell culture medium was changed. A, cell lysates were collected at 24 and 48 hours post-infection (hpi). Immunoblotting analysis was performed using rabbit monoclonal antibodies against focal adhesion kinase (FAK), cell division cycle 37 (Cdc37), β-actin, and a mouse monoclonal antibody against heat shock protein 90 (Hsp90); B, quantitative analysis of cellular proteins (FAK, Cdc37, Hsp90, and β-actin). Student <i>t</i>-test analysis was conducted to compare the quantitative expression of three proteins (FAK, Hsp90, and Cdc37) with β-actin at 24 and 48 hpi in rabies virus (RABV)-infected cells. The expression of all three proteins shows a significant increase, with the maximum expression is observed at 48 hpi. The infection does not affect the β-actin expression, which serves as a control (**** P < 0.0001). Neuro2a (N2a) cells were infected with the challenge virus standard-11 (CVS-11) for 2 hours [multiplicity of infection (MOI) = 1], and then the cell culture medium was changed. A, cell lysates were collected at 24 and 48 hours post-infection (hpi). Immunoblotting analysis was performed using rabbit monoclonal antibodies against focal adhesion kinase (FAK), cell division cycle 37 (Cdc37), β-actin, and a mouse monoclonal antibody against heat shock protein 90 (Hsp90); B, quantitative analysis of cellular proteins (FAK, Cdc37, Hsp90, and β-actin). Student <i>t</i>-test analysis was conducted to compare the quantitative expression of three proteins (FAK, Hsp90, and Cdc37) with β-actin at 24 and 48 hpi in rabies virus (RABV)-infected cells. The expression of all three proteins shows a significant increase, with the maximum expression is observed at 48 hpi. The infection does not affect the β-actin expression, which serves as a control (**** P < 0.0001).](https://services.brieflands.com/cdn/serve/3170b/f2196162b5e45b416d561c484d6aa16f7dabb583/jjm-160281-i002-F2-preview.webp)
![A, Western blot analysis of neuro2a (N2a) cells. In lane 1, normal N2a cells were infected with challenge virus standard-11 (CVS-11) [multiplicity of infection (MOI) = 1] and exposed to 1 μM PF-573,228 for 24 hours. Lane 2 showed the same cells 48 hours post-infection (hpi). Focal adhesion kinase (FAK) expression was completely inhibited by PF-573,228, a FAK inhibitor. However, no changes were observed in the expression heat shock protein 90 (Hsp90) and cell division cycle 37 (Cdc37). β-actin is considered a control protein; B, quantitative analysis of FAK, Cdc37, Hsp90, and β-actin in cells infected with CVS-11 and exposed to PF-573,228 (1 μM) at 24 and 48 hpi. The Hsp90 and Cdc37 expressions were compared with β-actin expression. Significant changes were observed in Hsp90 at 24 hpi, while changes occurred for Cdc37 at 48 hpi (* P < 0.05). PF-573,228, a kinase inhibitor, blocked FAK at 24 and 48 hpi, but it does not affect Hsp90, Cdc37, or β-actin. The higher expression of these two proteins at 48 hpi compared to 24 hpi was due to the prolonged presence of the rabies virus (RABV) infection. A, Western blot analysis of neuro2a (N2a) cells. In lane 1, normal N2a cells were infected with challenge virus standard-11 (CVS-11) [multiplicity of infection (MOI) = 1] and exposed to 1 μM PF-573,228 for 24 hours. Lane 2 showed the same cells 48 hours post-infection (hpi). Focal adhesion kinase (FAK) expression was completely inhibited by PF-573,228, a FAK inhibitor. However, no changes were observed in the expression heat shock protein 90 (Hsp90) and cell division cycle 37 (Cdc37). β-actin is considered a control protein; B, quantitative analysis of FAK, Cdc37, Hsp90, and β-actin in cells infected with CVS-11 and exposed to PF-573,228 (1 μM) at 24 and 48 hpi. The Hsp90 and Cdc37 expressions were compared with β-actin expression. Significant changes were observed in Hsp90 at 24 hpi, while changes occurred for Cdc37 at 48 hpi (* P < 0.05). PF-573,228, a kinase inhibitor, blocked FAK at 24 and 48 hpi, but it does not affect Hsp90, Cdc37, or β-actin. The higher expression of these two proteins at 48 hpi compared to 24 hpi was due to the prolonged presence of the rabies virus (RABV) infection.](https://services.brieflands.com/cdn/serve/3170b/b7f3e67bd952132b2d9a190330c6274bf809fb48/jjm-160281-i003-F3-preview.webp)

![A, cell division cycle 37 (Cdc37) overexpression in challenge virus standard-11 (CVS-11) infected cells; lane 1, neuro2a (N2a) cells were transfected with a pCMV-Script empty vector and then infected with the rabies virus (RABV) [multiplicity of infection (MOI) = 1] for 24 hours. P protein and Cdc37 expression levels were observed to be normal. Lane 2, cells were transfected with pCMV-Script-Cdc37 and then inoculated with the virus. Western blot analysis of the cell lysate displayed distinct bands of Cdc37 and P protein 48 hours post-infection (hpi). The high expression of the P protein was due to the overexpression of Cdc37. Lane 3, N2a cells were treated with PF-573,228 (1 μM) two hours after virus inoculation and detected 48 hpi. The inhibitor did not affect the expression of Cdc37 protein. Due to the inhibitor of focal adhesion kinase (FAK), a decrease in the expression of the P protein was observed, facilitated by heat shock protein 90 (Hsp90) and Cdc37 chaperones; B, β-actin is considered a control protein. Quantitative analysis of Cdc37 overexpression and β-actin, in cells infected with CVS-11 and exposed to PF-573,228 (1 μM) at 48 hpi. Lane 1, normal N2a cells were transfected with the pCMV-Script empty vector before RABV infection. Western blot analysis of cell lysate at 24 hpi showed significant differences between β-actin and Cdc37. The + sign indicates the presence and the - sign indicates the absence of Cdc37 (** P < 0.01). Lane 2, cells were transfected with pCMV-Script-Cdc37, and cell lysate blot analysis showed significant differences between β-actin and Cdc37 (** P < 0.01) 48 hpi. Lane 3, N2a cells were infected with the RABV, exposed to PF-573,228 (1 μM) for two hours, and checked 48 hpi. Although it did not affect the expression of the P protein, it caused a significant difference between β-actin and Cdc37 (** P < 0.01). β-actin is considered a control protein. A, cell division cycle 37 (Cdc37) overexpression in challenge virus standard-11 (CVS-11) infected cells; lane 1, neuro2a (N2a) cells were transfected with a pCMV-Script empty vector and then infected with the rabies virus (RABV) [multiplicity of infection (MOI) = 1] for 24 hours. P protein and Cdc37 expression levels were observed to be normal. Lane 2, cells were transfected with pCMV-Script-Cdc37 and then inoculated with the virus. Western blot analysis of the cell lysate displayed distinct bands of Cdc37 and P protein 48 hours post-infection (hpi). The high expression of the P protein was due to the overexpression of Cdc37. Lane 3, N2a cells were treated with PF-573,228 (1 μM) two hours after virus inoculation and detected 48 hpi. The inhibitor did not affect the expression of Cdc37 protein. Due to the inhibitor of focal adhesion kinase (FAK), a decrease in the expression of the P protein was observed, facilitated by heat shock protein 90 (Hsp90) and Cdc37 chaperones; B, β-actin is considered a control protein. Quantitative analysis of Cdc37 overexpression and β-actin, in cells infected with CVS-11 and exposed to PF-573,228 (1 μM) at 48 hpi. Lane 1, normal N2a cells were transfected with the pCMV-Script empty vector before RABV infection. Western blot analysis of cell lysate at 24 hpi showed significant differences between β-actin and Cdc37. The + sign indicates the presence and the - sign indicates the absence of Cdc37 (** P < 0.01). Lane 2, cells were transfected with pCMV-Script-Cdc37, and cell lysate blot analysis showed significant differences between β-actin and Cdc37 (** P < 0.01) 48 hpi. Lane 3, N2a cells were infected with the RABV, exposed to PF-573,228 (1 μM) for two hours, and checked 48 hpi. Although it did not affect the expression of the P protein, it caused a significant difference between β-actin and Cdc37 (** P < 0.01). β-actin is considered a control protein.](https://services.brieflands.com/cdn/serve/3170b/d701ce09711e308f33c952360858cc62e72b425e/jjm-160281-i006-F6-preview.webp)
![Heat shock protein 90 (Hsp90) knockdown and rabies infection; lane 1, neuro2a (N2a) cells were transfected with a scramble vector and infected with rabies virus (RABV) [multiplicity of infection (MOI) = 1]. Western blot analysis of cell lysate at 24 hours post-infection (hpi) showed that the expressions of Hsp90 and P protein were normal. Lane 2, the lack of the Hsp90 band was due to cell transfection with pEGFP-C1-ShHsp90 for 24 hours followed by infection with challenge virus standard-11 (CVS-11) for 48 hpi. The low expression of the P protein is due to the normal expression of focal adhesion kinase (FAK) and cell division cycle 37 (Cdc37). Lane 3, cells were transfected with pEGFP-C1-ShHsp90 for 24 hours, infected with the virus for two hours, treated with PF-573,228 (1 μM) for two hours, and then checked at 48 hpi. The very low expression of the P protein is also due to the additional influence of the Cdc37, as the inhibitor blocked FAK, and Hsp90 was knocked down by shRNA. β-actin is considered a control protein. Heat shock protein 90 (Hsp90) knockdown and rabies infection; lane 1, neuro2a (N2a) cells were transfected with a scramble vector and infected with rabies virus (RABV) [multiplicity of infection (MOI) = 1]. Western blot analysis of cell lysate at 24 hours post-infection (hpi) showed that the expressions of Hsp90 and P protein were normal. Lane 2, the lack of the Hsp90 band was due to cell transfection with pEGFP-C1-ShHsp90 for 24 hours followed by infection with challenge virus standard-11 (CVS-11) for 48 hpi. The low expression of the P protein is due to the normal expression of focal adhesion kinase (FAK) and cell division cycle 37 (Cdc37). Lane 3, cells were transfected with pEGFP-C1-ShHsp90 for 24 hours, infected with the virus for two hours, treated with PF-573,228 (1 μM) for two hours, and then checked at 48 hpi. The very low expression of the P protein is also due to the additional influence of the Cdc37, as the inhibitor blocked FAK, and Hsp90 was knocked down by shRNA. β-actin is considered a control protein.](https://services.brieflands.com/cdn/serve/3170b/f41c7ab4ddd5202244dc5e83c2ce1d38541eae13/jjm-160281-i007-F7-preview.webp)
![Cell division cycle 37 (Cdc37) knockdown and rabies infection; lane 1, cells were transfected with a scramble vector and infected with challenge virus standard-11 (CVS-11) [multiplicity of infection (MOI) = 1], and the expressions of Cdc37 and P protein were normal. Lane 2, the lack of a Cdc37 band was due to cell transfection with pCDH-CMV-MCS-ShCdc37 for 24 hours and infection with the virus for 48 hours post-infection (hpi). The expression of the P protein is due to the normal expression of focal adhesion kinase (FAK) and Hsp 90. Lane 3, cells were transfected with pCDH-CMV-MCS-ShCdc37 for 24 hours, infected with the virus for two hours, and then treated with 1 μM PF-573,228. The low expression of P protein is also due to the additional influence of heat shock protein 90 (Hsp90). This was caused by the inhibition of FAK and the knockdown of Cdc37. β-actin is considered a control protein. Cell division cycle 37 (Cdc37) knockdown and rabies infection; lane 1, cells were transfected with a scramble vector and infected with challenge virus standard-11 (CVS-11) [multiplicity of infection (MOI) = 1], and the expressions of Cdc37 and P protein were normal. Lane 2, the lack of a Cdc37 band was due to cell transfection with pCDH-CMV-MCS-ShCdc37 for 24 hours and infection with the virus for 48 hours post-infection (hpi). The expression of the P protein is due to the normal expression of focal adhesion kinase (FAK) and Hsp 90. Lane 3, cells were transfected with pCDH-CMV-MCS-ShCdc37 for 24 hours, infected with the virus for two hours, and then treated with 1 μM PF-573,228. The low expression of P protein is also due to the additional influence of heat shock protein 90 (Hsp90). This was caused by the inhibition of FAK and the knockdown of Cdc37. β-actin is considered a control protein.](https://services.brieflands.com/cdn/serve/3170b/3a5141207a191ac5b6d2379da5342ad8fcb83fb3/jjm-160281-i008-F8-preview.webp)