1. Background
Cystic fibrosis (CF) is a recessive autosomal disorder characterized by defective mucus secretion within the respiratory, digestive, and reproductive systems. Over 90% of individuals with CF have a chromosome 7 long arm defect, which leads to a deletion of phenylalanine at position 508 in the CFTR channel protein sequence (1, 2). This channel is located in epithelial tissues and is a chloride channel, which facilitates the outflow of chloride and water from the cell (3). This channel plays a role not only in epithelial surface homeostasis regulation but also in the regulation of other chloride and organic anion transporters, such as sodium and glutathione (4).
People with CF exhibit an insensitivity of the epithelial membrane to chloride ions. It is impermeable, which results in the imbalance between Cl ion extrusion due to CFTR and Na ion influx due to ENaC, ultimately resulting in the deficiency of fluid volume on the epithelial surface (5), which results in the secretion of thick mucous membranous tissue (3, 6). This causes the attachment and colonization of some bacteria on the thick and sticky mucus, especially in the respiratory tract, which is associated with chronic infections, progressive diseases, and even airway obstruction and often death in patients with CF (7, 8).
The sweat test is the most common test in the diagnosis of CF patients. This test measures the amount of sodium chloride (salt) in the sweat. In CF patients, the chloride ion concentration in sweat is greater than 60 mmol/L (9). The CF is a genetic disorder with a reported prevalence of approximately 1 in 2,500 to 3,500 births in the United States. However, its prevalence varies significantly across different ethnic groups, with lower rates observed among African Americans (1 in 17,000) and Asians (1 in 31,000) (10). The average life expectancy for individuals with CF is around 30 years, although advancements in therapeutic and maintenance interventions have contributed to an increase in life expectancy in recent years.
The spectrum of infectious agents in CF patients is relatively limited and primarily includes bacteria, viruses, and fungi. Among these, Staphylococcus aureus and Haemophilus influenzae are the most significant microbial agents, often colonizing patients early in life (11). Following these, bacteria such as Pseudomonas aeruginosa, P. cepacia, Stenotrophomonas maltophilia, Alcaligenes xyloxidans, and non-tuberculous mycobacteria are commonly identified in adulthood (11). Additionally, fungal agents such as Aspergillus fumigatus and Candida species are also prevalent in CF patients (12).
Pseudomonas aeruginosa is considered the most critical pathogen in CF, particularly due to its mucoid alginate-producing strains, which are highly resistant and challenging to eradicate from CF patients (13, 14). Currently, there is no definitive cure for CF, and treatment strategies primarily focus on managing symptoms resulting from CFTR deficiency. These include physical therapy to facilitate mucus clearance, antibiotic therapy for respiratory infections, and anti-inflammatory treatments such as macrolides (15). The prolonged and continuous use of antibiotics in pediatric patients with CF has contributed to the emergence and spread of antibiotic-resistant strains of microorganisms. This growing resistance poses significant challenges to effective treatment and long-term disease management. Therefore, understanding the current microbial profile and resistance patterns is essential.
2. Objectives
This study aimed to comprehensively identify the common microbial pathogens found in children diagnosed with CF and to evaluate their resistance and susceptibility patterns to commonly used antibacterial agents. By analyzing these patterns, the study seeks to provide insights that can support more effective, targeted, and sustainable antibiotic therapy in this vulnerable population.
3. Methods
3.1. Study Design
This study employed a cross-sectional design to investigate microbial organisms and antibacterial sensitivity and resistance patterns in children with CF. The study population consisted of 99 children with CF who were referred to the clinic of Ali Ebn Abitalib Hospital in Zahedan between 2023 and 2024. The hospital serves as a major referral center for CF patients in the region, making it an appropriate setting for this study. Children with a confirmed diagnosis of CF based on clinical and laboratory criteria, and availability of complete and relevant information on antibiotic resistance and sensitivity profiles, were included in the study. Children who did not meet these criteria were excluded from the study to ensure the accuracy and reliability of the findings.
3.2. Data Collection
For this retrospective study, we analyzed medical records of pediatric CF patients, focusing on microbiological culture results, antibiotic susceptibility patterns, and demographic data. To identify the bacterial causes of pharyngeal infections and evaluate antibiotic resistance, we examined throat swab samples collected from these patients. During sample collection, clinicians carefully obtained throat swabs using sterile cotton swabs, avoiding contact with the tongue, cheeks, and teeth to prevent contamination. These swabs were promptly streaked onto blood agar (to isolate Gram-positive bacteria like Streptococcus pyogenes) and cchocolate agar (to support fastidious Gram-negatives like Haemophilus influenzae). The cultures were then incubated at 35 - 37°C for 24 - 48 hours in a 5% CO2 atmosphere. After incubation, we examined microbial growth based on colony morphology, hemolysis patterns, and Gram stain results. For antibiotic susceptibility testing, we used the Kirby-Bauer disk diffusion method on Mueller-Hinton agar, measuring inhibition zone diameters and interpreting them per CLSI guidelines. The results classified bacterial isolates as sensitive or resistant to the antibiotics included in our study.
3.3. Data Analysis
The data were analyzed using SPSS 26 (IBM Corp., Armonk, NY, USA) and presented by descriptive statistics, such as frequencies, percentages, means, and standard deviations, to summarize the demographic characteristics of the study population, the prevalence of microbial organisms, and antibiotic resistance patterns.
4. Results
In this study, 99 children with CF who visited Ali Asghar Clinic in Zahedan between 2023 and 2024 were evaluated for microbial organisms and their response to antibacterial treatments. The analysis, as presented in Table 1, showed that out of 99 samples, the prevalence of pathogens was highest in S. aureus, 31 (31.3%), followed by P. aeruginosa, 29 (29.3%), and Acinetobacter, 6 (6.1%), respectively, while normal (no growth) cultures were 33 (33.3%). The table also revealed that P. aeruginosa was more prevalent in children over 10 years old (32.6%) compared to those under 10 years old (26.8%). In contrast, S. aureus was more common in children under 10 years old (37.5%) than in those over 10 years old (23.3%).
The proportion of normal cultures (no bacterial growth) was higher in children over 10 years old (39.5%) than in those under 10 years old (28.6%). Additionally, P. aeruginosa was more frequently observed in boys (35.7%) than in girls (24.6%), while S. aureus was equally distributed between boys and girls (35.7% and 28.1%, respectively). Acinetobacter, a group of bacteria, had the lowest frequency among the samples. The proportion of normal cultures was higher in girls (43.9%) than in boys (19.0%).
| Category | Pseudomonas aeruginosa | Staphylococcus aureus | Acinetobacter | Normal (No Growth) | Total |
|---|---|---|---|---|---|
| Age group (y) | |||||
| < 10 | 15 (26.8) | 21 (37.5) | 4 (7.1) | 16 (28.6) | 56 100) |
| > 10 | 14 (32.6) | 10 (23.3) | 2 (4.7) | 17 (39.5) | 43 (100) |
| Gender | |||||
| Girls | 14 (24.6) | 16 (28.1) | 2 (3.5) | 25 (43.9) | 57 (100) |
| Boys | 15 (35.7) | 15 (35.7) | 4 (9.5) | 8 (19.0) | 42 (100) |
| Total | 29 (29.3) | 31 (31.3) | 6 (6.1) | 33 (33.3) | 99 (100) |
a Values are expressed as No. (%).
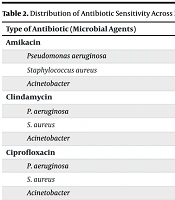
Table 2 highlighted the antibiotic susceptibility patterns of the identified pathogens. P. aeruginosa showed the highest susceptibility to ciprofloxacin (72.4%) and tobramycin (65.5%), making these the most effective antibiotics against this pathogen. However, it demonstrated no susceptibility to clindamycin and vancomycin, rendering these ineffective. Moderate susceptibility was observed for amikacin and gentamicin (62.1% each), suggesting they could serve as secondary treatment options. For S. aureus, vancomycin (74.2%) was the most effective antibiotic, underscoring its critical role in treating infections caused by this pathogen.
Moderate susceptibility was noted for ciprofloxacin (54.8%) and cotrimoxazole (38.7%), while most other antibiotics showed very low susceptibility (≤ 6.7%), limiting their utility. In the case of Acinetobacter, the highest susceptibility was observed for ciprofloxacin and imipenem (66.7% each), making them the preferred choices. No susceptibility was found for clindamycin, cotrimoxazole, and vancomycin, indicating these were ineffective. Moderate susceptibility was seen for amikacin, ceftazidime, gentamicin, and tobramycin (33.3% each), suggesting they may serve as alternative options in specific cases.
| Type of Antibiotic (Microbial Agents) | Yes | No |
|---|---|---|
| Amikacin | ||
| Pseudomonas aeruginosa | 18 (62.1) | 11 (37.9) |
| Staphylococcus aureus | 2 (6.7) | 29 (93.3) |
| Acinetobacter | 2 (33.3) | 4 (66.7) |
| Clindamycin | ||
| P. aeruginosa | 0 (0) | 29 (100) |
| S. aureus | 8 (25.8) | 23 (74.2) |
| Acinetobacter | 0 (0) | 6 (100) |
| Ciprofloxacin | ||
| P. aeruginosa | 21 (72.4) | 7 (27.6) |
| S. aureus | 17 (54.8) | 14 (45.2) |
| Acinetobacter | 4 (66.7) | 2 (33.3) |
| Ceftazidime | ||
| P. aeruginosa | 16 (55.2) | 13 (44.8) |
| S. aureus | 2 (6.5) | 29 (93.5) |
| Acinetobacter | 2 (33.4) | 4 (66.6) |
| Cotrimoxazole | ||
| P. aeruginosa | 1 (3.4) | 28 (96.6) |
| S. aureus | 12 (38.7) | 19 (61.3) |
| Acinetobacter | 0 (0) | 6 (100) |
| Gentamicin | ||
| P. aeruginosa | 18 (62.1) | 11 (37.9) |
| S. aureus | 2 (6.5) | 29 (93.5) |
| Acinetobacter | 2 (33.3) | 4 (66.7) |
| Vancomycin | ||
| P. aeruginosa | 0 (0) | 29 (100) |
| S. aureus | 23 (74.2) | 8 (25.8) |
| Acinetobacter | 0 (0) | 6 (100) |
| Tobramycin | ||
| P. aeruginosa | 19 (65.5) | 10 (34.5) |
| S. aureus | 1 (3.2) | 30 (96.8) |
| Acinetobacter | 2 (33.3) | 4 (66.7) |
| Imipenem | ||
| P. aeruginosa | 11 (37.9) | 18 (62.1) |
| S. aureus | 2 (6.5) | 29 (93.5) |
| Acinetobacter | 4 (66.7) | 2 (33.3) |
a Values are expressed as No. (%).
Table 3 outlined the resistance patterns of the pathogens. P. aeruginosa exhibited the highest resistance to imipenem (51.7%) and gentamicin (37.9%), indicating significant challenges in treating infections with these antibiotics. Moderate resistance levels were observed for amikacin (34.5%) and ceftazidime (20.7%), suggesting limited efficacy. Lower resistance rates were noted for tobramycin (17.2%), cotrimoxazole (10.3%), and ciprofloxacin (10.3%), while no resistance was detected against tetracycline, penicillin, erythromycin, and clindamycin, highlighting their potential utility. For S. aureus, extremely high resistance rates were observed for erythromycin (90.3%) and clindamycin (64.5%), underscoring their ineffectiveness. Moderate resistance was noted for cotrimoxazole (32.3%), ciprofloxacin (32.3%), and penicillin (25.8%), indicating limited therapeutic options. Low resistance levels were found for tetracycline (9.7%) and tobramycin (3.2%), while no resistance was detected against imipenem, gentamicin, ceftazidime, and amikacin, making these antibiotics more reliable for treatment.
| Type of Antibiotic (Microbial Agents) | Yes | No |
|---|---|---|
| Tetracycline | ||
| Pseudomonas aeruginosa | 0 (0) | 29 (100) |
| Staphylococcus aureus | 3 (9.7) | 28 (90.3) |
| Acinetobacter | 0 (0) | 6 (100) |
| Tobramycin | ||
| P. aeruginosa | 5 (17.2) | 24 (82.8) |
| S. aureus | 1 (3.2) | 30 (96.8) |
| Acinetobacter | 4 (66.7) | 2 (33.3) |
| Imipenem | ||
| P. aeruginosa | 15 (51.7) | 14 (48.3) |
| S. aureus | 0 (0) | 31 (100) |
| Acinetobacter | 2 (33.3) | 4 (66.7) |
| Penicillin | ||
| P. aeruginosa | 0 (0) | 29 (100) |
| S. aureus | 8 (25.8) | 23 (74.2) |
| Acinetobacter | 0 (0) | 6 (100) |
| Cotrimoxazole | ||
| P. aeruginosa | 3 (10.3) | 26 (89.7) |
| S. aureus | 10 (32.3) | 21 (67.7) |
| Acinetobacter | 3 (50) | 3 (50) |
| Erythromycin | ||
| P. aeruginosa | 0 (0) | 29 (100) |
| S. aureus | 28 (90.3) | 3 (9.7) |
| Acinetobacter | 0 (0) | 6 (100) |
| Gentamicin | ||
| P. aeruginosa | 11 (37.9) | 18 (62.1) |
| S. aureus | 0 (0) | 31 (100) |
| Acinetobacter | 4 (66.7) | 2 (33.3) |
| Clindamycin | ||
| P. aeruginosa | 0 (0) | 29 (100) |
| S. aureus | 20 (64.5) | 11 (35.5) |
| Acinetobacter | 0 (0) | 6 (100) |
| Ciprofloxacin | ||
| P. aeruginosa | 3 (10.63) | 26 (89.37) |
| S. aureus | 10 (32.3) | 21 (67.7) |
| Acinetobacter | 1 (16.7) | 5 (83.3) |
| Ceftazidime | ||
| P. aeruginosa | 6 (20.7) | 23 (79.3) |
| S. aureus | 0 (0) | 31 (100) |
| Acinetobacter | 4 (66.7) | 2 (33.3) |
| Amikacin | ||
| P. aeruginosa | 10 (34.5) | 19 (65.5) |
| S. aureus | 0 (0) | 31 (100) |
| Acinetobacter | 4 (66.7) | 2 (33.3) |
a Values are expressed as No. (%).
In the case of Acinetobacter, high resistance rates were observed for tobramycin, gentamicin, clindamycin, ceftazidime, and amikacin (66.7% each), reflecting significant treatment challenges. Moderate resistance was noted for imipenem (33.3%) and cotrimoxazole (50%), while low resistance was observed for ciprofloxacin (16.7%). No resistance was detected against tetracycline, penicillin, and erythromycin, suggesting these antibiotics may serve as potential alternatives in specific cases.
Table 4 summarized the antibiotic sensitivity and resistance patterns of P. aeruginosa, S. aureus, and Acinetobacter spp. P. aeruginosa showed sensitivity to amikacin, ciprofloxacin, ceftazidime, gentamicin, and tobramycin. S. aureus was sensitive to ciprofloxacin, cotrimoxazole, and vancomycin but resistant to clindamycin and imipenem. Acinetobacter spp showed sensitivity to amikacin, ceftazidime, and imipenem, but resistance to clindamycin, cotrimoxazole, and vancomycin.
| Types of Antibiotic | Microbial Agent | |||||
|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | Staphylococcus aureus | Acinetobacter spp | ||||
| Sensitivity | Resistance | Sensitivity | Resistance | Sensitivity | Resistance | |
| Amikacin | ✔ | ✔ | ✔ | |||
| Clindamycin | ✔ | ✔ | ✔ | |||
| Ciprofloxacin | ✔ | ✔ | ✔ | |||
| Ceftazidime | ✔ | ✔ | ✔ | |||
| Cotrimoxazole | ✔ | ✔ | ✔ | |||
| Gentamicin | ✔ | ✔ | ||||
| Vancomycin | ✔ | ✔ | ✔ | |||
| Tobramycin | ✔ | ✔ | ✔ | |||
| Imipenem | ✔ | ✔ | ✔ | |||
5. Discussion
This study examined microbial agents and antibiotic resistance patterns in children with CF. The findings revealed that S. aureus was the most commonly identified microorganism, followed by P. aeruginosa and Acinetobacter. A portion of the patients also showed no bacterial growth in their cultures. These findings align with those reported by Kodori et al. (15) in Tehran and Khan et al. (16) in Pakistan. The prevalence of S. aureus as the primary microbial agent in bacterial infections among children with CF in southeastern Iran highlights the need for clinicians to consider this in treatment plans. Only one-third of the children had normal microbial cultures, indicating a high percentage of bacterial infections. Studies by Erfanimanesh et al. (17) in Tehran, Fazeli et al. (18) in Isfahan, and Perikleous et al. (19) in Greece demonstrated that these children are prone to bacterial infections, which may be due to a weakened immune system and living conditions. Therefore, preventive measures by healthcare providers should be strongly recommended to avoid further complications in these children. A study conducted in India reported bacterial growth in 246 samples (55%), with 48 samples (19.5%) exhibiting mixed infections, particularly in older children.
The highest positive culture rate (62.5%) was observed in children aged 3 - 6 months, with P. aeruginosa (52.6%) and S. aureus being the most frequently identified organisms. These findings are somewhat consistent with the microbial distribution observed in the present study. In the current study, P. aeruginosa was more prevalent among children over ten years of age (32.6%), whereas S. aureus was more frequently detected in children under ten years of age (37.5%). However, statistical analysis using the chi-square test revealed no significant association between microbial distribution and age.
This result is in accordance with the findings of Perikleous et al. (19) in Greece, where, despite the highest prevalence occurring in the 1 - 12-year age group, no statistically significant differences were observed in the age distribution of microbial agents. These findings suggest that the main factor influencing microbial infections in children with CF may not be their age, but rather how well they follow preventive measures and the general health challenges linked to the disease, which make them more vulnerable to infections. This result is consistent with the study by Erfanimanesh et al. (17) in Tehran but not with Perikleous et al. (19) in Greece, where bacterial infections were more prevalent in boys than in girls, indicating greater susceptibility in boys, especially at younger ages. Discrepancies with some studies may be due to study methods, sample sizes, and diagnostic laboratory conditions, warranting further research in this area.
The highest sensitivity to antibiotics such as amikacin, clindamycin, ciprofloxacin, ceftazidime, cotrimoxazole, gentamicin, vancomycin, tobramycin, and imipenem was observed in P. aeruginosa (62.1%), S. aureus (25.8%), P. aeruginosa (72.4%), P. aeruginosa (52.2%), S. aureus (38.7%), P. aeruginosa (62.1%), S. aureus (74.2%), P. aeruginosa (65.5%), and Acinetobacter (66.7%) respectively. This result partially aligns with the distribution of microbial agents found in the study by Bashir et al. (20) in India. In that study, P. aeruginosa strains were highly sensitive to all aminoglycosides, piperacillin-tazobactam, and polymyxin, while Enterococcus strains showed similar sensitivity to methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) to vancomycin, linezolid, and teicoplanin.
Overall, 61% of cultures were positive, with S. aureus being the most common organism, and P. aeruginosa isolates being largely sensitive to aminoglycosides, carbapenems, and polymyxin. In a study by Kodori et al. (15) in Tehran, P. aeruginosa was the most common bacterium isolated after S. aureus, with antibiotic sensitivity tests showing the highest resistance to piperacillin-tazobactam (11.7%) and the lowest resistance to gentamicin (2.3%). Moreover, 83.4% of S. aureus strains were sensitive to methicillin, while 16.6% were methicillin-resistant. According to this study, P. aeruginosa was the predominant pathogen in children with CF, which is not entirely consistent with the present study's findings.
The present study also indicated that the highest antibiotic resistance to tetracycline, tobramycin, imipenem, penicillin, cotrimoxazole, erythromycin, clindamycin, ciprofloxacin, ceftazidime, and amikacin was observed in S. aureus (9.7%), Acinetobacter (66.7%), P. aeruginosa (51.7%), S. aureus (25.8%), Acinetobacter (50%), S. aureus (90.3%), Acinetobacter (66.7%), S. aureus (64.5%), S. aureus (32.3%), Acinetobacter (66.7%), and Acinetobacter (66.7%) respectively. This result does not align with the study by Baghbani-Arani et al. (21), which may be due to differences in sample collection methods, equal selection of samples from both genders, and reporting statistics without distinguishing microbial agents. In the mentioned study, 35% of strains exhibited multidrug resistance, and most strains (96%) were resistant to rifampin, with the highest sensitivity to streptomycin (96%), imipenem (93%), and meropenem (94%).
In a study by Gautam et al. (22), children whose initial cultures were positive for P. aeruginosa showed mixed microbial cultures in 55% of subsequent cultures. P. aeruginosa infections were most sensitive to ciprofloxacin (89%) and piperacillin-tazobactam (88%). Additionally, 38% of S. aureus strains were methicillin-resistant. In a study by Emerson et al. (23) conducted in the United States, sputum samples from 267 participants across 33 CF centers were analyzed. A total of 656 P. aeruginosa isolates were identified from 253 culture-positive participants. The study found a significant increase in the prevalence of tobramycin-resistant (11.8% vs. 30.4%) and amikacin-resistant (24.2% vs. 42.7%) P. aeruginosa strains over time. However, ciprofloxacin resistance remained stable (34.4% vs. 33.6%, P = 0.81).
The study also explored links between recent antibiotic use and resistance patterns, revealing that intravenous carbapenem exposure was significantly associated with resistance to aztreonam, meropenem, and multidrug resistance. Additionally, the prevalence of S. aureus, MRSA, S. maltophilia, and Achromobacter xylosoxidans increased in the more recent cohort. While this study provides valuable insights into microbial colonization and antibiotic resistance in CF children, several limitations should be acknowledged. The retrospective design may introduce biases related to incomplete or inconsistent record-keeping, and being a single-center study may limit the generalizability of the findings to other populations or settings. Finally, the sample size, though adequate for preliminary analysis, may not be sufficient to detect rare microbial organisms or resistance patterns.
5.1. Conclusions
The study concluded that S. aureus and P. aeruginosa are the most common microbial agents in CF, with only one-third showing normal microbial cultures. This highlights a high prevalence of bacterial infections, likely due to weakened immunity and environmental factors. Age and gender did not significantly affect microbial distribution, emphasizing the need for universal preventive measures. Antibiotic sensitivity tests showed P. aeruginosa was highly sensitive to ciprofloxacin and ceftazidime, while S. aureus exhibited significant resistance to erythromycin and clindamycin. Clinicians should focus on regular microbial monitoring and tailored antibiotic therapy, prioritizing infection prevention and patient education to reduce bacterial infections and resistance.