1. Background
Approximately one-quarter of the world's population has either the active or latent form of tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis. The TB, which used to be the leading cause of death from a single infectious agent for many years, has fallen to second place in the last three years with the emergence of SARS-CoV-2. One of the biggest challenges in the fight against TB today is antimicrobial resistance. The emergence of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) complicates the fight further. Globally, in 2023, 159,684 people were diagnosed with MDR-TB, and 28,982 people with pre-XDR-TB/XDR-TB (1). Another significant impact of TB is its synergy with HIV-infected or immunocompromised individuals. It is known that individuals with HIV are 50 times more likely to develop TB (2). In 2023, a total of 436,805 TB cases were reported among people living with HIV worldwide. Overall, the percentage of people who test positive for HIV and are newly diagnosed with TB has reportedly fallen globally over the past 10 years (1).
There are many drugs available today to treat TB. However, none of them offer a rapid, definitive, and effective treatment option. The main reason for this is that the TB bacillus becomes resistant to these drugs very quickly. Although most of the drug resistance occurs due to spontaneous chromosomal mutations, the presence of the TB bacillus's relatively thick, complex cell wall and the activity of various efflux pumps also contribute to this (3, 4). Rapidly increasing antimicrobial resistance emphasizes the urgency of the need for new alternative drug candidates. There are also some limitations in the pharmaceutical field for TB drug development. Some of these include the identification and verification of new drug candidates, long-term clinical phase trials, economic costs, side effects, toxicities, and treatment durations. Despite these necessary but problematic stages, there are promising new TB drug candidates that have been developed recently (5).
Considering the difficulties in developing new anti-TB drugs, “drug repositioning” strategies have recently been adopted to identify new therapeutic uses for existing approved drugs. This popular approach has additional advantages such as relatively low resource investment, elucidated pharmacokinetic and pharmacodynamic studies, reliability, and a known toxicity profile (6, 7). It is also a very attractive approach as a drug identification method in the fight against TB.
Clofazimine, levofloxacin, moxifloxacin, meropenem, and linezolid are successful examples of drug repositioning strategies against TB. These drugs are already approved by the WHO for use in the treatment of MDR-TB (8, 9). Meropenem is a member of the carbapenem family of the beta-lactam class and has been included in the TB treatment regimen through the drug repositioning strategy. Beta-lactam antibiotics are important antimicrobial drugs that target the peptidoglycan synthesis process, which plays a critical role in the synthesis of the bacterial cell wall. TB bacilli are intrinsically resistant to β-lactams, mainly due to the low permeability of the bacillus cell wall and the presence of a constitutive β-lactamase that hydrolyzes most penicillins and cephalosporins (10, 11).
Beta-lactam antibiotics were not initially an option for TB treatment due to their low anti-TB activity. However, their potential has been re-evaluated with the emergence of beta-lactamase enzyme inhibitors. Ceftazidime/avibactam (CAV) and amoxicillin/clavulanic acid (AMO/CLAV) are among the successful examples of beta-lactam and beta-lactamase inhibitor combinations (12, 13). The CAV is currently used successfully in the clinic for the treatment of gram-negative bacillus infections. The CAV combination is known to have a strong bactericidal effect against MDR and XDR-TB strains in vitro. At clinically accessible doses, it is seen as a promising strategy in treatment-resistant TB cases (14, 15).
The AMO/CLAV is frequently used in the treatment of most gram-negative and gram-positive bacterial infections. Methicillin-susceptible Staphylococcus aureus (MSSA), Neisseria species, community-acquired pneumonia, and group A streptococcal carriers are the agents for which this combination is used (16). In studies evaluating AMO/CLAV in terms of anti-TB activity, it showed a synergistic effect when combined with primary anti-TB drugs isoniazid (INH), rifampicin (RIF), and ethambutol (EMB), indicating that it may have potential for use in the TB treatment regimen (17, 18). Often known as a "drug of last resort", colistin (CS) is a polycationic antibiotic known for its activity against gram-negative bacteria, particularly Enterobacteriaceae. However, the fact that many gram-negative bacteria now possess CS resistance genes is a significant public health concern, reducing the number of effective antibiotics available. Although CS is known to have limited anti-TB activity, some studies have reported that it has a synergistic effect with anti-TB agents (19, 20).
None of these drug/drug combinations are currently part of the TB treatment regimen. However, in vitro studies have shown that they have the potential to be a life-saving and effective alternative for the treatment of TB patients. Much more research is needed on the conditions, with which drugs, at which doses, and in which patient groups they will be used in the clinic. However, first of all, the in vitro activities of these drugs against TB isolates should be confirmed with other studies.
2. Objectives
This study aimed to determine the antimicrobial activity of CAV, AMO/CLAV, and CS on M. tuberculosis isolates with different resistance profiles using the E-test (gradient diffusion) method.
3. Methods
3.1. Mycobacterium tuberculosis Isolates
All strains tested in the study were obtained from the culture collection of Akdeniz University, Faculty of Health Sciences, Research Laboratory-TB Research Unit (Antalya, Turkiye). All test isolates were identified by GeneXpert® MTB/RIF (Cepheid, USA). Five reference ATCC strains and 36 M. tuberculosis isolates were tested. The reference ATCC strains included M. tuberculosis ATCC 27294 (H37Rv) and it’s in vitro mutant strains: ATCC 35822 (INH-resistant), ATCC 35838 (RIF-resistant), ATCC 35820 [Streptomycin (STR)-resistant], and ATCC 35837 (EMB-resistant). A total of 41 isolates, including ATCC strains, were tested for AMO/CLAV (BioMerieux, France and Bioanalyse, Turkiye), 35 isolates for CAV (BioMerieux, France and Bioanalyse, Turkiye), and 20 isolates for CS (BioMérieux, France and Bioanalyse, Turkiye). Primary drug susceptibilities of all test isolates were previously confirmed with the BACTEC MGIT 960 system (Becton-Dickinson, USA). The resistance profiles of the tested isolates are shown in Table 1.
| Isolate | Results | Isolate | Results | Isolate | Results | Isolate | Results |
|---|---|---|---|---|---|---|---|
| ATCC 27294 | SSSS | C14 | RSSS | MDR-4 | SRRS | N2 | RRRR |
| ATCC 35822 | SRSS | C15 | SSSS | MDR-1 | SRRR | MDR-14 | RRRR |
| ATCC 35838 | SSRS | C18 | RSSS | N4 | RRRR | C4 | SRSR |
| ATCC 35820 | RSSS | 18-AYC | SRRS | MDR-38 | SRRR | N14 | SRRS |
| ATCC 35837 | SSSR | 8-AYC | SRRR | K2443 | RRRS | N16 | SRRS |
| C2 | RRSS | 40-AYC | RRRR | K8704 | RRRS | MDR-46 | SRRS |
| C3 | SRSS | C1 | RRSS | 2013-10 | RRRR | N18 | SRRR |
| C5 | RSSS | C8 | RRRS | MDR-19 | RRRR | MDR-43 | SRRS |
| C6 | RRSS | C10 | RRSS | N10 | SRRS | - | - |
| C12 | SRSS | N12 | RRRR | A14 | RSRR | - | - |
| C13 | RSSS | N3 | SRRS | N5 | SRRR | - | - |
Abbreviations: S, sensitive; R, resistance.
a Primary anti-tuberculosis drugs (streptomycin, isoniazid, rifampicin, ethambuol).
3.2. Preparation of Mycobacterial Inoculum
In this study, where infectious particles were used at high concentrations, all experimental steps were carried out in a BSL-3 laboratory, in a class II type B microbiological biosafety cabinet, using personal protective equipment (3M Versaflo, TR-300), in accordance with the recommended biosafety principles (21). Bacterial inoculum were prepared using fresh bacterial cultures grown in Lowenstein-Jensen (LJ) medium, as previously recommended by Yildirim et al. (22). McFarland no ≥ 3 inoculums of all isolates were prepared using a McFarland densitometer (BIOSAN Medical-Biological Research & Technologies, Riga, Latvia).
3.3. Preparation of Medium
The E-test (gradient diffusion) method was performed on Middlebrook 7H11 Agar (BD-Difco™, USA). The medium was prepared according to the manufacturer's recommendations. After the medium was sterilized by autoclaving, it was cooled to 50 - 55 ºC, and 10% oleic acid, albumin, dextrose, catalase (OADC) was added as a supplement. The medium was distributed into sterile disposable petri dishes with a diameter of 90 mm to a depth of 4 - 4.5 mm. The prepared agar plates were stored at +4 ºC until use (23).
3.4. E-test Strips
All E-test strips used in the study were obtained from the manufacturers (bioMerieux, France and Bioanalyse, Turkiye). The strips were stored at -20°C until used.
3.5. Implementation of E-test (Gradient Diffusion) Method
Mycobacterial inoculums prepared with McFarland > 3 turbidity were spread on the surface of Middlebrook 7H11 agar plates in three directions with sterile swabs. Agar plates were incubated at 37°C for approximately 1 hour to absorb the suspension spread on the surface. Then, an E-test strip was placed in the center of each agar plate. An agar plate without an E-test strip was simultaneously prepared as a growth control plate for each test isolate. Agar plates were placed in ziplock plastic bags and incubated at 37°C in an environment of 5- 10% CO2. The results were evaluated on days 14 - 21 of incubation. The minimum inhibitory concentration (MIC) value was determined as the antibiotic concentration at the point where the inhibition ellipse formed around the E-test strips intersected the strip (23).
3.6. Nitrocefin Disk Test for Detection of β-lactamase
Beta-lactamase detection was performed for 16 M. tuberculosis isolates and 5 ATCC strains using nitrocefin disks (Bioanalyse, Turkey) according to the manufacturer's recommendations. For each isolate, 1 nitrocefin disk was placed into a sterile tube and moistened with 500 µL of sterile distilled water. Fresh cultures of M. tuberculosis isolates were suspended in the solution in the tube. All tubes were incubated for 30 - 45 min at room temperature. At the end of incubation, yellow color formation was evaluated as "beta-lactamase negative" and red color formation was evaluated as "beta-lactamase positive". Beta-lactamase producing strain S. aureus ATCC 29213 and beta-lactamase negative strain Moraxella catarrhalis Moraxella catarrhalis ATCC 25238 were used as positive and negative controls, respectively (24).
3.7. Statistical Analysis
Descriptive statistical analyses were performed to evaluate the MIC values obtained for AMO/CLAV, CAV, and CS. For each antibiotic, basic statistical parameters including mean, standard deviation, minimum, maximum, and interquartile ranges (25th, 50th, and 75th percentiles) were calculated. In addition, MIC50 and MIC90 values were determined, representing the concentrations required to inhibit 50% and 90% of the tested isolates, respectively. All statistical calculations were conducted using the Python programming language with the aid of the pandas and numpy libraries.
4. Results
The MIC values determined for AMO/CLAV acid are shown in Table 2, the MIC values determined for CAV are shown in Table 3, and the MIC values determined for CS are shown in Table 4. Nitrocefin disk results are shown in Table 5. Agar plates containing AMO/CLAV test strips of some selected isolates are shown in Figure 1, agar plates containing CAV test strips in Figure 2, and agar plates containing CS test strips in Figure 3. In this study, the MIC values of AMO/CLAV, CAV, and CS were assessed against M. tuberculosis isolates. For AMO/CLAV, MIC values ranged from 0.016 µg/mL to 256 µg/mL across 41 isolates, with a mean of 149.7 µg/mL and a standard deviation of 121.8. Both the MIC50 and MIC90 values were calculated as 256 µg/mL, indicating that a large proportion of the isolates exhibited high levels of resistance.
| Mycobacterium tuberculosis | MIC | Isolate | MIC | Isolate | MIC | Isolate | MIC | Isolate | MIC |
|---|---|---|---|---|---|---|---|---|---|
| ATCC 27294 b | > 256 | C2 b | > 256 | 18-AYC b | ≤ 0.016 | MDR-1 | > 256 | N5 | > 256 |
| ATCC 35822 b | 12 | C3 b | 0.75 | 8-AYC b | > 256 | N4 | 12 | N2 | > 256 |
| ATCC 35838 b | 3 | C5 b | > 256 | 40-AYC b | > 256 | MDR-38 | > 256 | MDR-14 | > 256 |
| ATCC 35820 b | 32 | C6 b | > 256 | C1 b | 1.5 | K2443 | > 256 | C4 b | > 256 |
| ATCC 35837 b | 4 | C12 b | 0.064 | C8 b | > 256 | K8704 | > 256 | N14 | > 256 |
| - | - | C13 b | 48 | C10 b | 1 | 2013-10 | 8 | N16 | ≤ 0.016 |
| - | - | C14 b | 0.38 | N12 | > 256 | MDR-19 | > 256 | MDR-46 | ≤ 0.016 |
| - | - | C15 b | 128 | N3 | > 256 | N10 | > 256 | N18 | ≤ 0.016 |
| - | - | C18 b | 0.38 | MDR-4 | > 256 | A14 | > 256 | MDR-43 | > 256 |
Abbreviations: MIC, minimum inhibitory concentration.
a Values are expressed as µg/mL.
b Isolates with beta-lactamase activity determined.
| Mycobacterium tuberculosis | MIC | Isolate No | MIC | Isolate No | MIC | Isolate No | MIC | Isolate No | MIC |
|---|---|---|---|---|---|---|---|---|---|
| ATCC 27294 b | ≤ 0.016 | C2 b | ≤ 0.016 | 18-AYC b | ≤ 0.016 | K8704 | ≤ 0.016 | N8 | > 256 |
| ATCC 35822 b | ≤ 0.016 | C3 b | ≤ 0.016 | 8-AYC b | ≤ 0.016 | N10 | ≤ 0.016 | N14 | > 256 |
| ATCC 35838 b | 0.023 | C5 b | ≤ 0.016 | 40-AYC b | > 256 | N5 | > 256 | N16 | > 256 |
| ATCC 35820 b | ≤ 0.016 | C12 b | ≤ 0.016 | C1 b | ≤ 0.016 | N2 | ≤ 0.016 | MDR-46 | > 256 |
| ATCC 35837 b | ≤ 0.016 | C13 b | ≤ 0.016 | C8 b | ≤ 0.016 | MDR-6 | > 256 | N18 | ≤ 0.016 |
| - | - | C14 b | ≤ 0.016 | C10 b | ≤ 0.016 | N9 | ≤ 0.016 | MDR-43 | > 256 |
| - | - | C15 b | ≤ 0.016 | N15 | > 256 | MDR-14 | > 256 | - | - |
| - | - | C18 b | ≤ 0.016 | N4 | > 256 | C4 b | > 256 | - | - |
Abbreviations: MIC, minimum inhibitory concentration.
a Values are expressed as µg/mL.
b Isolates with beta-lactamase activity determined.
| Mycobacterium tuberculosis | MIC | Isolate No | MIC | Isolate No | MIC | Isolate No | MIC |
|---|---|---|---|---|---|---|---|
| ATCC 27294 | > 256 | C2 | 64 | C13 | 128 | 8-AYC | > 256 |
| ATCC 35822 | 48 | C3 | 0.125 | C14 | 0.25 | 40-AYC | > 256 |
| ATCC 35838 | > 256 | C5 | > 256 | C15 | 48 | C1 | 0.125 |
| ATCC 35820 | 128 | C6 | 0.25 | C18 | 0.047 | C8 | 0.125 |
| ATCC 35837 | > 256 | C12 | 0.125 | 18-AYC | 0.047 | C10 | 0.5 |
Abbreviations: MIC, minimum inhibitory concentration.
a Values are expressed as µg/mL.
| Isolate | Result | Isolate | Result | Isolate | Result | Isolate | Result |
|---|---|---|---|---|---|---|---|
| ATCC 27294 | Negative | C3 | Positive | C15 | Negative | C8 | Negative |
| ATCC 35822 | Positive | C5 | Positive | C18 | Positive | C10 | Negative |
| ATCC 35838 | Positive | C6 | Positive | 18-AYC | Negative | C4 | Positive |
| ATCC 35820 | Positive | C12 | Positive | 8-AYC | Negative | - | - |
| ATCC 35837 | Positive | C13 | Positive | 40-AYC | Positive | - | - |
| C2 | Positive | C14 | Positive | C1 | Positive | - | - |
a Beta-lactamase activity.
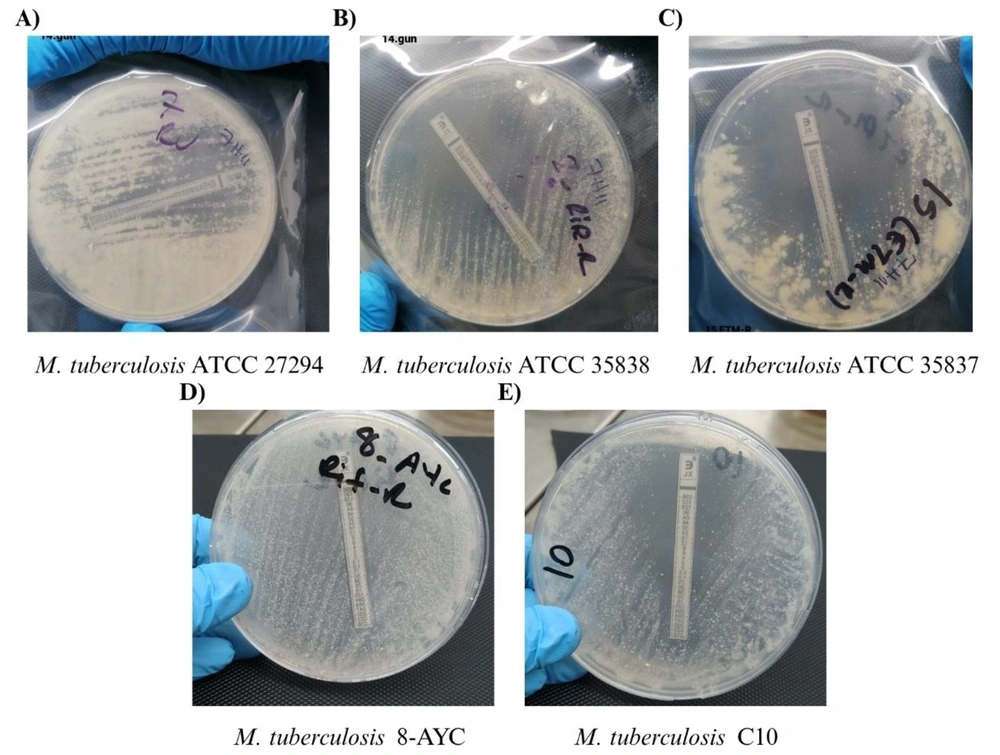
Agar plates containing amoxicillin/clavulanic acid (AMO/CLAV) test strips of some selected Mycobacterium tuberculosis isolates; A, agar plate containing AMO/CLAV test strip of M. tuberculosis ATCC 27294; B, agar plate containing AMO/CLAV test strip of M. tuberculosis ATCC 35838; C, agar plate containing AMO/CLAV test strip of M. tuberculosis ATCC 35837; D, agar plate containing AMO/CLAV test strip of M. tuberculosis 8-AYC; E, agar plate containing AMO/CLAV test strip of M. tuberculosis C10.
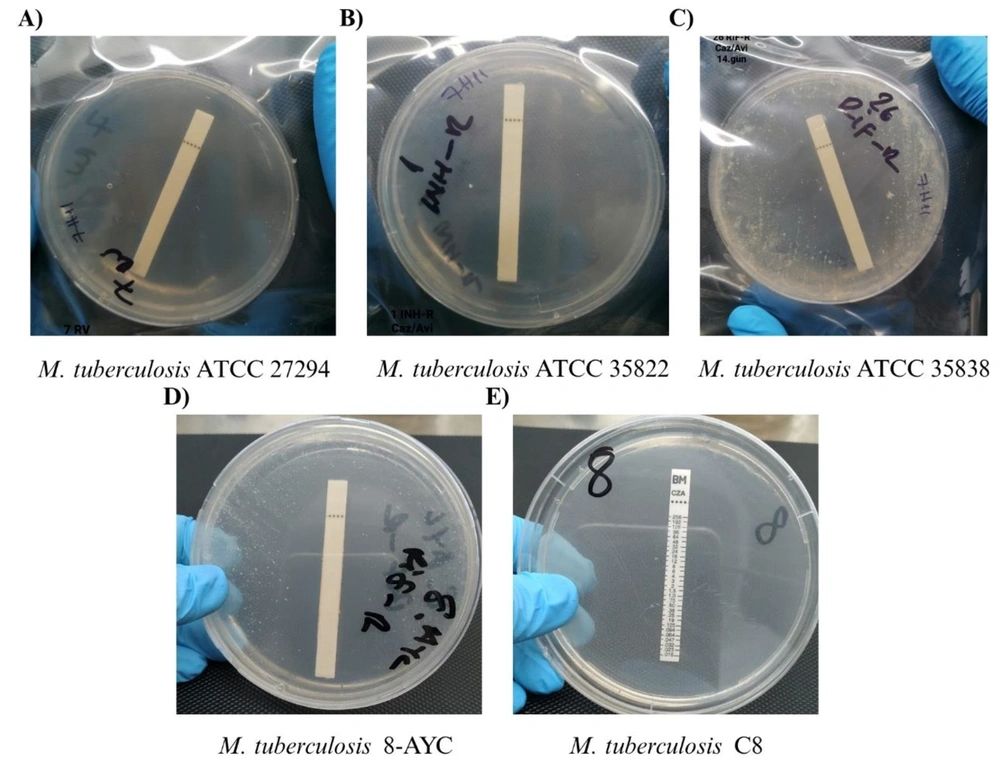
Agar plates containing ceftazidime/avibactam (CAV) test strips of some selected Mycobacterium tuberculosis isolates; A, agar plate containing CAV test strip of M. tuberculosis ATCC 27294; B, agar plate containing CAV test strip of M. tuberculosis ATCC 35822; C, agar plate containing CAV test strip of M. tuberculosis ATCC 35838; D, agar plate containing CAV test strip of M. tuberculosis 8-AYC; E, agar plate containing CAV test strip of M. tuberculosis C8.
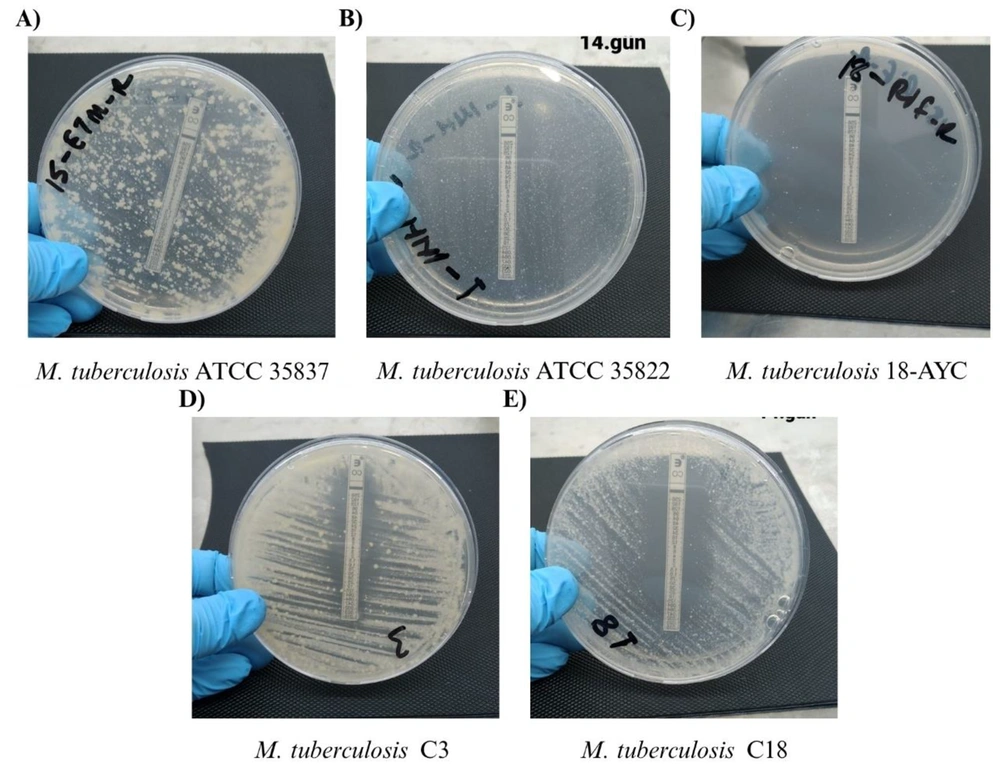
Agar plates containing colistin (CS) test strips of some selected Mycobacterium tuberculosis isolates; A, agar plate containing CS test strip of M. tuberculosis ATCC 35837; B, agar plate containing CS test strip of M. tuberculosis ATCC 35822; C, agar plate containing CS test strip of M. tuberculosis 18-AYC; D, agar plate containing CS test strip of M. tuberculosis C3; E, agar plate containing CS test strip of M. tuberculosis C18.
For CAV, 35 isolates were analyzed, and MIC values also ranged from 0.016 µg/mL to 256 µg/mL. The mean MIC was 73.2 µg/mL with a standard deviation of 115.6. While the MIC50 was notably low at 0.016 µg/mL, the MIC90 reached 256 µg/mL, reflecting a bimodal distribution where some isolates were highly susceptible and others were resistant. In the case of CS, MIC values obtained from 20 isolates ranged between 0.047 µg/mL and 256 µg/mL. The mean MIC was 97.7 µg/mL, and the standard deviation was 110.4. The MIC50 and MIC90 values were calculated as 48 µg/mL and 256 µg/mL, respectively, suggesting a moderate to high resistance pattern among the tested isolates.
Overall, these findings reveal a wide variation in the susceptibility profiles of M. tuberculosis isolates and underscore the importance of MIC50 and MIC90 metrics in evaluating potential therapeutic efficacy. The relatively high MIC values observed for many isolates suggest limited effectiveness for these antibiotics in certain clinical contexts and highlight the necessity for cautious interpretation in resistance management.
5. Discussion
The TB, a global public health problem, will always require new drug candidates as long as it cannot be eradicated due to its ability to rapidly acquire antimicrobial resistance. In addition to developing new drug candidates, creating new areas of use for currently used and approved drugs seems to be a more advantageous strategy (25, 26). Beta-lactams are a broad class of antimicrobial agents and have shown their potential effects with the emergence of beta-lactamase inhibitors. These beta-lactam and beta-lactamase inhibitor drug combinations have also attracted significant attention in terms of their anti-TB activities. The potential of incorporating this drug class into TB treatment regimens through a “drug repositioning” strategy and the synergistic effects observed in combination with other anti-TB agents in in vitro studies are quite promising (27-29).
In the study conducted by Desphande et al. (14), the activity of ceftazidime and its avibactam combination was evaluated against MDR-TB and extensively XDR-TB isolates using the Hollow-Fiber System Model of TB (HFS-TB). Neither ceftazidime nor avibactam alone killed TB bacilli, while CAV killed TB bacilli with an Emax of 4.19-7.05 Log10 CFU/mL. In this case, it showed equal activity to RIF and better than INH and pyrazinamide (PZA). The CAV administered with human-like pharmacokinetics showed greater bactericidal activity than first-line drugs in monotherapy and combination. In the same time period, in the same HFS-TB, INH and RIF had a killing effect of < 2.0 Log10 CFU/mL, while CAV had a killing effect of 6.0 Log10 CFU/mL in only 7 days. In addition, the study showed that CAV killed a subpopulation of intracellular M. tuberculosis and had a high level of intracellular penetration. When the sterilizing effect of CAV was compared with the INH-RIF-PZA triple combination, it was seen that the killing effect of the three-drug combination treatment was more successful than CAV monotherapy, but the same killing effect was reached with CAV on the 42nd day of the experiment (14).
Srivastava et al. evaluated the efficacy of ceftriaxone, another cephalosporin with a longer half-life, due to the short half-life of CAV, in the HFS-TB model. It was tested with CAV as a source of avibactam as a β-lactamase inhibitor. The MIC of ceftriaxone for M. tuberculosis H37Ra was 16 mg/L without avibactam, but decreased to 4 mg/L in the presence of 15 mg/L avibactam. Then, the MIC range of ceftriaxone in combination with 15 mg/L avibactam was found to be between 0.5 and 32 mg/L against 30 clinical isolates. In summary, the ceftriaxone-CAV dual β-lactam combination was shown to be more lethal than either cephalosporin "monotherapy" in the HFS-TB model (30).
Srivastava et al. also investigated the use of cefazolin for the treatment of MDR-TB in children using pharmacokinetic and pharmacodynamic principles. Avibactam was reported to reduce cefazolin MICs by five tube dilutions. The cefazolin-avibactam combination showed a maximum killing of 4.85 log10 CFU/mL in an intracellular HFS-TB model over 28 days. The distribution of MICs among MDR-TB isolates suggests that cefazolin has the potential to be developed for the treatment of TB (31). In these studies, it is seen that other cephalosporins, apart from ceftazidime, have potential for use in terms of anti-TB effects when combined with avibactam. Since avibactam is not commercially available alone, its formulations with CAV were used. Therefore, double cephalosporin combinations including CAV need to be investigated further.
In our study, CAV MICs were determined as 0.023 µg/mL for ATCC 35838 among 5 reference ATCC strains, while they were ≤ 0.016 µg/mL for all other strains. The CAV MICs were determined as ≤ 0.016 µg/mL in 18 of the 30 tested M. tuberculosis isolates, while they were > 256 µg/mL in 12 of them. Of the 18 isolates with CAV MICs of ≤ 0.016 µg/mL, 8 were MDR-TB isolates, 9 had different resistance profiles, and 1 was an M. tuberculosis isolate susceptible to all primary drugs. Of the 12 isolates with CAV MIC > 256 µg/mL, 11 were MDR-TB isolates and 1 was only STR-EMB resistant. In total, the CAV combination was found to be highly effective in 65.71% of the isolates tested (23 out of 35). According to the nitrocefin disk result, only 2 of the 14 isolates that were beta-lactamase positive had CAV MIC > 256 µg/mL, 11 had ≤ 0.016 µg/mL, and 1 had 0.023 µg/mL. As expected, all isolates with negative beta-lactamase activity had MIC ≤ 0.016 µg/mL.
Another successful example of the combination of this class of beta-lactam antibiotics with inhibitory agents is AMO/CLAV acid. In vitro studies highlight the potential of this drug combination for the treatment of TB. Pagliotto et al. tested AMO/CLAV alone and in combination with other primary anti-TB drugs against 23 M. tuberculosis isolates using the reassuring drug combination microtiter test (REDCA). In the study, MIC values for AMO/CLAV ranged from 2 - 16 mg/L. It was reported that the AMO/CLAV+INH combination showed a synergistic effect in 8 isolates, while AMO/CLAV+RIF and AMO/CLAV+EMB combinations showed synergy in 19 isolates. It is suggested that this effect, especially seen on MDR-TB isolates, may be an alternative for resistant TB treatment (17).
In the study conducted by Cynamon and Palmer, the in vitro activity of the combination of amoxicillin and clavulanic acid against M. tuberculosis isolates was evaluated. When used alone, amoxicillin was able to inhibit only 26% (4/15) of the isolates and did not show any bactericidal effect. The combination of amoxicillin and clavulanic acid showed a bactericidal effect on 14 of 15 isolates tested at a concentration of 4 µg/mL amoxicillin and 2 µg/mL clavulanate. The addition of clavulanic acid suppressed the beta-lactamase activity of M. tuberculosis strains and thus increased the activity of amoxicillin (32).
In the study conducted by Segura et al., amoxicillin, carbenicillin, cefotaxime, ceftriaxone, aztreonam, and combinations of these antibiotics with clavulanate (2:1 ratio) were tested. All M. tuberculosis isolates in the study showed resistance to beta-lactam antibiotics by producing beta-lactamase. When used alone, amoxicillin was not effective in both susceptible and MDR-TB strains (MIC > 64 µg/mL), but its combination with clavulanate significantly reduced the MIC value. When the AMO/CLAV combination was used, the MIC value decreased to 16 µg/mL in susceptible strains, while this value was determined as 32 µg/mL in MDR strains. This study shows that beta-lactamase activity plays an important role in beta-lactam antibiotic resistance in M. tuberculosis strains. Particularly, the AMO/CLAV combination showed promising efficacy on both susceptible and MDR-TB strains (33).
In our study, a total of 41 isolates, including ATCC strains, were tested for AMO/CLAV. MIC values were > 256 µg/mL in 23 of the isolates, ≤ 0.016 µg/mL in 4 of them, and in the range of 0.064 - 128 µg/mL in the others. Among the isolates with MIC values > 256 µg/mL, 17 were MDR isolates, and all 4 isolates with ≤ 0.016 µg/mL were also MDR isolates. The AMO/CLAV combination was found to be ineffective in 56.09% (23 out of 41 isolates) of the tested isolates. According to nitrocefin disk results, only 5 of 15 beta-lactamase positive isolates had MIC > 256 µg/mL, while the remaining 10 isolates had MIC values ranging from 0.064 - 48 µg/mL. Of the 6 beta-lactamase negative isolates, MIC was determined as > 256 µg/mL in 3, 128 µg/mL in 1, and ≤ 0.016 µg/mL and 1 µg/mL in the remaining 2 isolates, respectively.
In our study, we also investigated the anti-TB activity of CS, known as the drug of last resort, in addition to beta-lactam combinations. Bax et al. investigated the activity of CS and anti-TB drug combinations against M. tuberculosis strains in vitro. The aim of the study was to investigate whether CS increases the effectiveness of anti-TB drugs such as INH, RIF, and amikacin by increasing M. tuberculosis cell wall permeability. Although CS alone has a limited effect on M. tuberculosis, it showed a synergistic effect in M. tuberculosis populations with high metabolic activity when used in combination with INH and amikacin. In contrast, no synergy was observed in its combination with RIF. The researchers suggest that inhalation administration of CS may help achieve high local concentrations and may be used as a new strategy in TB treatment (34).
In the study conducted by van Breda et al. (20), they investigated the in vitro effect of CS methane sulfonate (CMS) on M. tuberculosis. The aim of the study was to determine the MIC and minimum bactericidal concentration (MBC) values of CMS and to investigate the effect of CMS in the presence of pulmonary surfactant (PS). In addition, the synergistic effect of the combination of CMS with INH and RIF was also evaluated. In the study conducted using the M. tuberculosis H37Ra strain, the MIC value of CMS was determined as 16 mg/L and the MBC value as 256 mg/L. In the presence of PS, the MIC value of CMS increased 8-fold to 128 mg/L.
This situation is explained by the complex formation of CMS with PS. The combination of CMS and INH provided a reduction of 2 log10 CFU/mL (> 99%), and this combination showed the strongest synergistic effect. The combination of CMS and RIF was found to be ineffective. The researchers emphasize that CMS should be evaluated as a potential agent in the treatment of MDR-TB, but it needs to be supported by clinical studies (20).
Metabolomic analyses by Koen et al. have shown that CMS treatment disrupts the cell membrane of M. tuberculosis and causes changes in cell wall synthesis. CMS causes a change in the energy metabolism of M. tuberculosis and increases fatty acid synthesis to repair the cell wall. It is also suggested that CMS may facilitate the entry of other antibiotics into the cell by disrupting the hydrophobic barrier. Researchers suggest that CMS can be used as a potential adjuvant agent in the treatment of MDR-TB (35).
In our study, a total of 20 M. tuberculosis isolates were tested to determine the anti-TB activity of CS. While the MIC value was > 256 µg/mL in 6 of the isolates, it was found to be in the range of 0.047 - 0.5 µg/mL in 9 of them. In the remaining 5 isolates, it varied between 48 - 128 µg/mL. In this study, we report that the effectiveness of CAV, AMO/CLAV, and CS varied in the tested M. tuberculosis isolates. Especially, the AMO/CLAV combination was found to be ineffective in 56% of the isolates. This combination may have low effectiveness when used alone, but it may have a synergistic effect in combination with other primary drugs.
In addition, since MIC values were determined only with the E-test method in our study, it is necessary to test it with different methods and conduct in vitro studies including more isolates to confirm its anti-TB activity. In our study, the CAV combination was found to be more effective than the AMO/CLAV combination. The MIC value was determined as ≤0.016 µg/mL in 65.71% of the tested isolates. The CAV combination showed its sterilizing effect in all of these isolates. Current studies suggest that the inclusion of CAV in the TB treatment regimen will have significant contributions when used together with other antibiotics due to its sterilizing effect. The effectiveness of CS shows a wide distribution among isolates. These results suggest that treatment options may be limited, especially in MDR-TB strains, and alternative combinations with various antibiotics should be evaluated.
5.1. Conclusions
In our study, the CAV combination was found to be more effective than the AMO/CLAV combination. Current studies suggest that the inclusion of CAV in the TB treatment regimen will have significant contributions when used together with other antibiotics due to its sterilizing effect.