1.Background
Helicobacter pylori has been associated with gastritis, peptic ulcers, and gastric carcinoma (1, 2). Although infection with H. pylori is a global health problem, its prevalence is different among countries (3). This difference reflects socioeconomic factors including large families, crowded living conditions, and poor sanitation (4), but ethnicity may contribute independently (5). It is now generally agreed that H. pylori infections are acquired during childhood or adolescence in developing as well as developed countries (6).
Despite its great prevalence in our country, epidemiologic studies of H. pylori infection is limited. A recent study performed by histopathology in Ardabil, north-west of Iran, revealed about 90% of H. pylori infection in the normal population, older than 40 years old (7). There are several techniques available for detection of H. pylori. There is evidence that infected individuals excrete H. pylori in feces, since the pathogen could be detected in stool specimens by an antigen enzyme immunoassay (EIA).
Many studies support the diagnostic value of H. pylori antigens in stool samples by enzyme immunoassay (EIA) (8).This test was approved in 1998 for both diagnosis and monitoring the response to treatment of H. pylori infection in adult patients (9). Recently, a study performed by Falsafi T et al. using stool test for detection of H. pylori antigen in children and adolescents from Tehran demonstrated a high prevalence of infection (70%) in cases with a family history of H. pylori infection or peptic ulcer disease (10).
2. Objectives
The availability of non-invasive antigen enzyme immunoassay (EIA) made it possible to carry out the present study to determine the prevalence rates of H. pylori infection in general population in Khuzestan province, south-west of Iran.
3. Patients and Methods
Population of Khuzestan province in south-west of Iran is around 4.5 million with various socioeconomic statuses. The area was visited and homes were chosen by cluster sampling according to study design and research methodology. We described the study to the habitants and after their agreement to cooperation, an enrollment date was fixed and all the family members present in that date were enrolled as subjects to the inclusion and exclusion criteria. Each family was provided with a consent form to be signed by parents. The study was approved by our Ethical Committee.
3.1. Inclusion Criteria
The studied individuals were healthy, aged 0-80 years, washed out for 30 days of any medication such as H2-receptor antagonists, acid pump inhibitors, non-steroidal anti-inflammatory drugs, or bismuth compounds that influenced H. pylori detection.
3.2. Data Collection
On the enrollment date, questionnaire forms were collected back and dates of birth and other data of the family members were noted. A unique identification (ID) number was assigned for each family member. All subjects were interviewed and personal, socioeconomic status, parents’ educational level, environmental, and geographical data were obtained. From September to October 2009, 861 persons were sampled in the province. Subjects were asked to collect a specimen from their first stool.
Patients with recent history of treatment for H. pylori infection or antibiotic use for other purposes were excluded. Stool samples were stored at -70 ºC until performing the test that was independently performed by the Tropical Disease Research Center laboratory. The stool specimens were analyzed for H. pylori antigen using HpSA immunoassay as described by its manufacturer. A commercial kit, STRA (Italy) was used. The fecal sample was mixed with 200 μL of the sample diluents. One drop of enzyme conjugates was added to the micro wells, which were incubated for 1 hour at room temperature and washed. The reaction was terminated with one drop of stop solution and the results were read by ELISA system. A particular cut-off value was considered positive as recommended by the manufacturer; the absorbance was read at both single (450-nm) and dual (450/630-nm) wavelengths. Positive and negative controls were run with each batch of samples. A positive test was indicated by a yellow color and a negative test was either colorless or faint yellow.
We analyzed the interview data and stool antigen tests by statistical package of WIN SPSS 15 and Stata 10; linear and logistic regression analyses were used as appropriate. Data were presented as proportions, mean ± 2 SE; the Student’s t-test was used for comparison of two means, and the chi-square test for multiple proportions.
4. Results
The overall HpSA positivity was (53.5% ,CI 95% = 50.2-56.84%).the mean age of infected cases was greater than that of non-infected ones (29.2 ± 1.54 years vs. 24.5 ± 1.8 years) (P > 0.001). HpSA positivity increased with age. The peak prevalence was reached in the 41–50 years age group (66%), with a relatively stable prevalence, thereafter. The specific prevalence of H. pylori positivity for each age group in the total sample is shown in Table 1.
| Age Group, y | HPSA Positive, No. (%) | Total HPSA Positive Cases, % |
|---|---|---|
| 0-10 | 53 (33) | 11.5 |
| 11-20 | 104 (53.6) | 22.6 |
| 21-30 | 119 (60.7) | 25.8 |
| 31-40 | 78 (59.1) | 17 |
| 41-50 | 58 (66) | 12.6 |
| 51-60 | 29 (59.2) | 6.3 |
| 60 < | 20 (48.8) | 4.3 |
Risk factors to H. pylori infection according to univariate analysis are shown in Table 2.
| Risk Factor | Crude Odds Ratio | P value |
|---|---|---|
| Age group, y | ||
| 0-10 | Reference group | P< 0.001 |
| 41-50 | 3.9 | P< 0.001 |
| Education | ||
| Middle school | Reference group | P< 0.001 |
| Illiterate | 1.6 | |
| Job | ||
| Student | Reference group | P< 0.001 |
| Farmer | 3.03 |
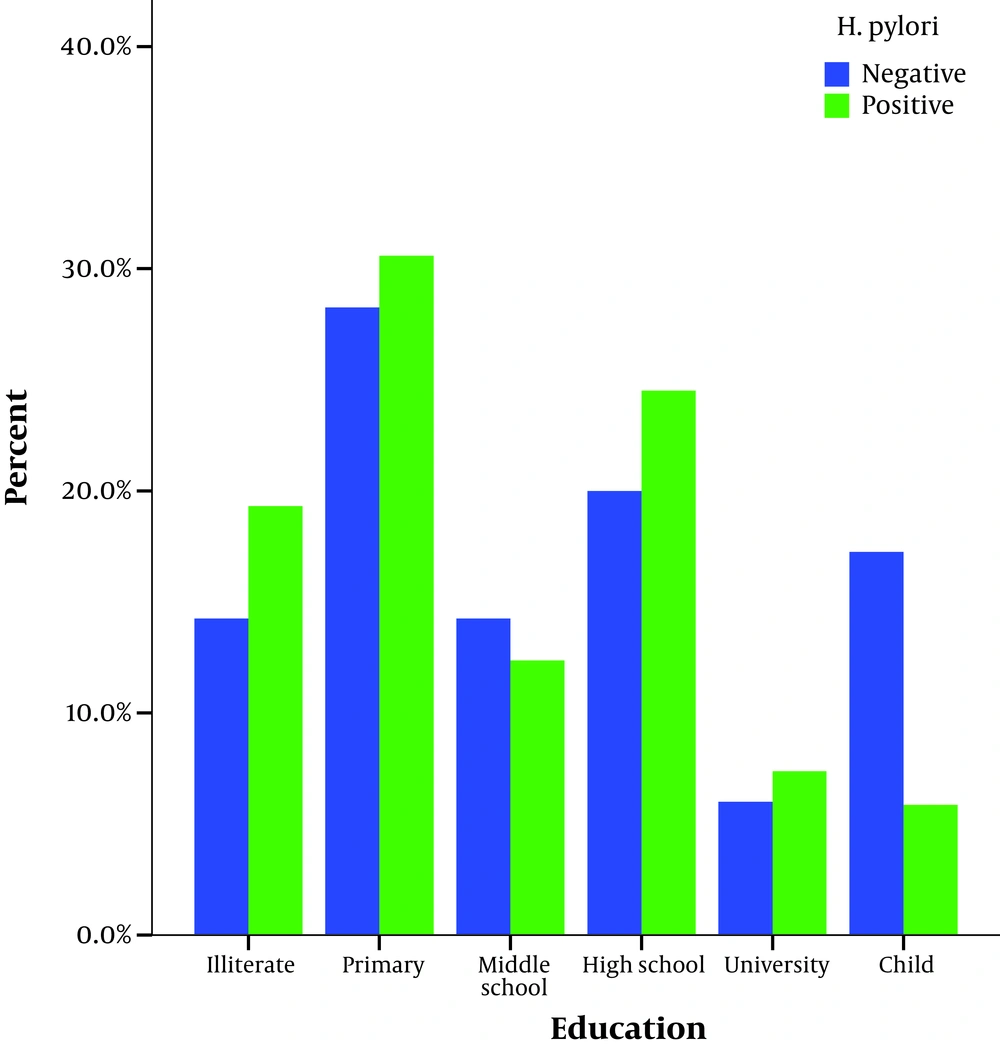
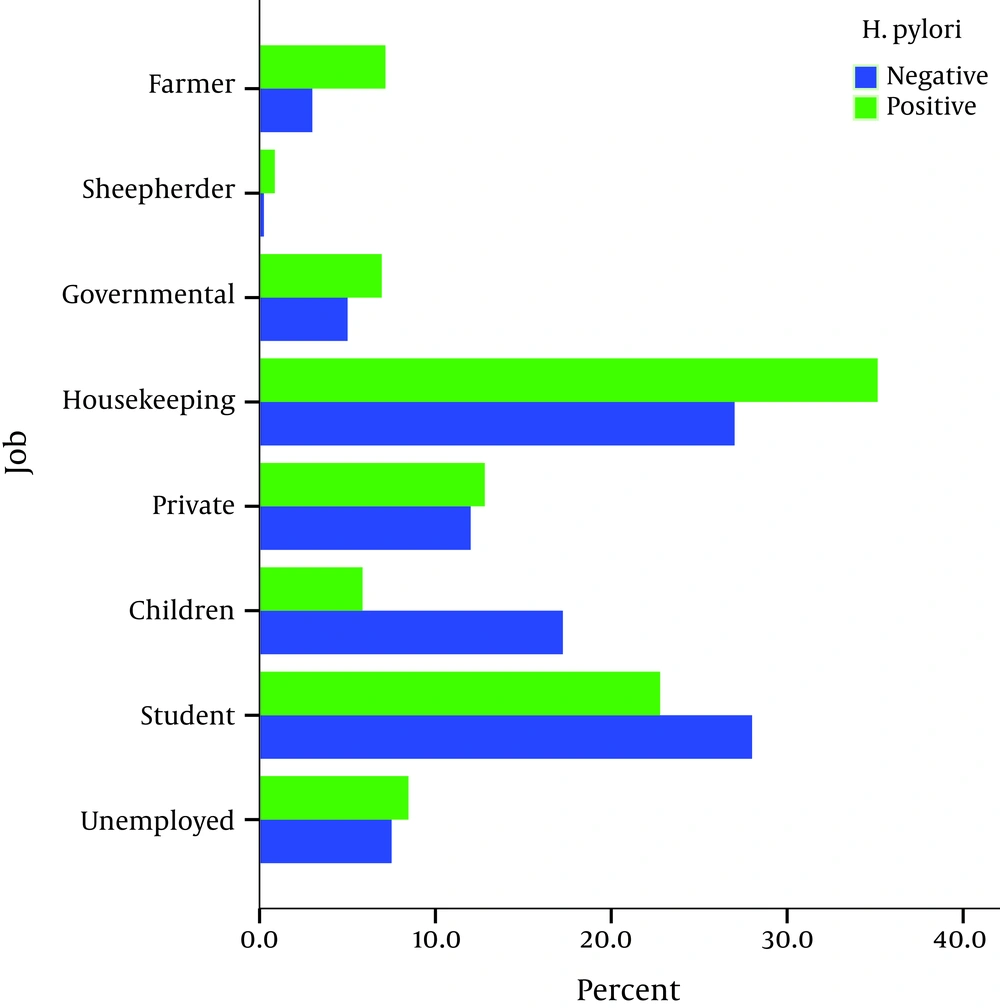
The infection was significantly associated with age, job, and lower educational level (lower high school level) at univariate analysis (P < 0.001) (Figures 1 and 2).
In relation to access to facilities like personal computer, electrical washing machine, or internet, there were no significant differences by H. pylori stool antigen status (P > 0.07). Additionally, there was no significant relationship between H. pylori infection and sex (male & female), previous hospital admission, gastrointestinal symptoms, and living area. (P > 0.05).
5. Discussion
H. pylori infection is thought to play an etiologic role in several gastro-duodenal diseases. In epidemiological studies, H. pylori antigens (HpSA) in stool could offer high sensitivity and specificity (9). HpSA is a noninvasive method for determining the presence of H. pylori organisms. This study showed high infection rates of H. pylori among adults, children, and teenagers living in our community. Overall, 50-57% of the study population was infected with H. pylori. HpSA positivity increased with age. The peak prevalence was reached in the 41–50 years age group (66%) with a relatively stable prevalence, thereafter. A similar trend has been observed in other countries, since the acquisition of H. pylori is known to occur mostly during the childhood.
This corresponds very closely to figures from other developing countries, e.g. 63% in Turkey and Tehran (10, 11), but lower than India (80%) (12), and Pakistan (79%) (13). The lower rate of infection among Iranian adults might be the result of frequent antibiotic usage among Iranian society or gradual improvement of socioeconomic status during the last two decades.
Our result is lower than that shown in previous studies in our country, i.e. 69.0% in Tehran and 79.4% in Kerman (14, 15). This may be due to the fact that those studies were not population-based; only volunteers or referrals to clinics had been examined. In addition, another reason for such differences is the method of H. pylori diagnosis in those populations that was H. pylori IgG antibodies. Among the noninvasive detection methods that are practical for population-based studies, the urea breath test (UBT) and stool antigen tests (HpSA) are considered as the most accurate, while serology is the least costly and most widely available. H. pylori seroprevalence studies most commonly used IgG which has the disadvantage of non-differentiation value between current and past infections; whereas H. pylori IgG antibodies often decline to negative levels once the infection has resolved, the frequency and timing of this occurrence differs substantially across populations (16).
Previous investigations have shown the importance of sex and age in the acquisition of H. pylori infection (17). Some studies have shown no sex difference in the prevalence of H. pylori (18, 19) but in a study in Iran, H. pylori prevalence was higher in males than in females (20).In our study there was no significant relationship between H. pylori infection and sex. We found an inverse relationship between H. pylori prevalence and educational level. H. pylori infection was higher in subjects of lower compared to those with higher educational levels. This difference was statistically significant (P < 0.001). In this study, high- educated participants had a lower frequency of H. pylori infection compared to those who were low-educated (7.4% vs 30.6%, respectively).
Low socioeconomic jobs were associated with a higher prevalence of H. pylori colonization in our studied population. This result could reflect the difference in the standards of living conditions between the two groups of studied population. It further supports the consensus that low socioeconomic status is associated with increase in prevalence of H. pylori infection (21). Regarding the job groups, our investigation found that jobs with poor hygiene were associated with a higher risk of acquiring H. pylori infection. For example, the study showed a higher prevalence of H. pylori infection among illiterate farmers than in educated professionals with odds ratio of 3.03 (P < 0.001). Consistent with other studies, we could not find any significant relationship between access to facilities of adulthood like personal computer, electrical washing machine, or internet and H. pylori infection rate (22).
The present study had limitations. Although the detection of H. pylori antigen in stool has a sensitivity and specificity of 90−95%, other tests (such as urease test and culture of the organism from gastric biopsies) or combination tests may have improved our detection rate (6, 9). Nevertheless, this study is the first in Khuzestan province, south-west of Iran and provide insights into the epidemiology of H. pylori in this area. In addition, longitudinal data on H. pylori-related clinical events such as peptic ulcer disease and gastric cancer would allow the assessment of the impact of H. pylori in our community.
To evaluate the accuracy of stool antigen test, an endoscopic evaluation and biopsy or a recently developed monoclonal antibody is useful. In this study, we did not have such control groups; this is a limitation in our study. The aim of this study was to estimate the prevalence of H. pylori infection in our area; therefore other studies are needed to reach to this goal. Other prospective cohort studies are required to clearly and define the aspects of H. pylori infection epidemiology in this area.
In conclusion, the study shows H. pylori infection is highly prevalent (57%) in Khuzestan province, south-west of Iran. Based on our findings, H. pylori infection rate obtained from this study is lower than those previously described, and correlates with increasing age, but not gender. Low educational level and the job group of the study subjects were found as the possible risk factors related to considerable rate of H. pylori infection among our community.