1. Background
Vulvovaginal candidiasis (VVC) or Candida vaginitis is a common fungal infection among adult women during reproductive ages. It has been estimated that 75% of all adult women experience at least one period of vulvovaginal candidiasis in their lifetime (1). fortunately the infection is rarely life threatening, whereas it is usually associated with such morbidities like discomfort, pain, sexual dysfunctions, vulvar dryness, cracks, itching, burning, soreness and finally health care costs (2-4). Known predisposing host factors, which include uncontrolled diabetes mellitus, using contraceptive, compromised immune system, neutropenia, pregnancy, hormone replacement therapy and broad-spectrum antibiotics are risk factors for VVC (1, 5).
Several reports have shown that prevalence of vulvovaginal candidiasis in Iran is remarkable and similar to other parts of the world (6-10). However, there are a few reports about susceptibility of vaginal isolates to antifungal agents in vitro circumstances. In addition some studies have shown that there are different results from treatment of vulvovaginal candidiasis (9, 11-15). There are also several reports indicating that resistance to antifungals, and infection recurrent is a serious problem among Iranian patients (11, 12, 14). In a study performed in Qazvin, authors believed that there is no significant difference between fluconazole and clotrimazole resistance in recurrent candidiasis (9). Prolonged therapy and increased use of antifungals for recurrent candidiasis are the most common risk factors for azoles resistance among Candida isolates from vulvovaginitis candidiasis patients. Azoles have the advantage of being taken orally, which increase their potency (2, 4).
The inappropriate use of antifungal drugs and introduction of over-the-counter antimycotics in countries worldwide predispose development of antifungal resistance (6). In a study conducted by Richter et al., fluconazole resistance was observed among 15.2% and 41.7% of vaginal isolates of C. glabrata and C. krusei, respectively (1). Whereas resistance to itraconazole was observed in non-albicans species, C. glabrata, C. parapsilosis, C. krusei, and S. cerevisiae isolates (1). In another study, vaginal isolates of Candida were more dose-dependent susceptible to nystatin and ketoconazole (16). Candida species are the normal microbiota within the oral cavity, gastrointestinal tracts, respiratory tracts, vaginal area and the mouth (4). The majority of cases of vulvovaginal candidiasis are caused by C. albicans, other etiologic agents are C. glabrata, C. tropicalis and C. krusei (1, 4, 17). However, Mohanty et al. has reported C. glabrata as the main etiology of vulvovaginal candidiasis (13).
2. Objectives
This study was carried out to determine susceptibility patterns of vaginal isolates of Candida species to eight antifungals including, clotrimazole, miconazole, itraconazole, fluconazole, ketoconazole, econazole, terbinafine, and nystatin.
3. Patients and Methods
3.1. Isolates and Identification
In the present study, 67 vulvovaginal isolates of Candida species were investigated for performing susceptibility tests. The isolates were kept in sterile distilled water at 4°C in the medical mycology laboratory of Ahvaz Jundishapur University of medical sciences. The most common isolate was C. albicans (53, 79.1%) followed by C. glabrata (8, 11.9%), C. tropicalis (4, 5.9%) and C. krusei (2, 2.9%). All isolates were re-identified using standard methods, CHROMagar Candida (CHROMagar Candida®, Paris, France) germ tube test and microscopic characteristics on corn meal agar (HiMedia, India). Identification was based on colonies producing a green coloration which were presumptively identified as C. albicans (17). Germ tube test, production of chlamydoconidia and growing at 45°C were also a confirmation for the isolates (17). C. glabrata produced pink colonies on CHROMagar Candida which microscopic characteristics on corn meal agar confirmed it (17). Dark blue coloration on CHROMagar Candida and microscopic features confirmed C. tropicalis. Pale pink and spread colonies on CHROMagar Candida and microscopic features on Cornmeal agar were identical for C. krusei.
3.2. Antifungal Disks
Paper disks containing clotrimazole at 50μg/disk, miconazole at 10μg/disk, itraconazole 50μg/disk, fluconazole 100μg/disk, ketoconazole 10μg/disk, econazole 10μg/disk, and ystatin 100U/disk were obtained from Liofilchem Bacteriology Products (Italy). Terbinafine disks were also prepared at 50μg/disk.
3.3. Test Method
A total of 67 Candida species were sub-cultured on Sabouraud's dextrose agar plates, SDA (Merck, Germany) and incubated at 37°C for 24h. A suspension of overnight cultures of C. albicans (53), C. glabrata (8) and C. tropicalis (4) and C. krusei (2) were prepared in sterile PBS. Turbidity was adjusted to 0.5 McFarland standard density resulting in an inoculum containing 1-5×106 CFU/ml. 25µl of suspension inoculated on SDA plates and rolled on the surface of the agar medium. Plates were dried for 15min at room temperature in laminar hood and then antifungal disks were placed on the inoculated agar with a forceps. The plates were incubated at 37°C for 24h, and then zone diameters were measured manually.
4. Results
In the present study susceptibility testing was performed on vaginal isolates of Candida collected during 2008 to 2009 from patients suspected to vulvovaginal candidiasis in Ahvaz, the capital city of Khuzestan. Criteria for susceptibility to used antifungal drugs have been summarized in Table 1 (17-19). In the present study, several topical and systemic antifungal drugs were evaluated against 53 isolates of C. albicans, 8 C. glabrata, 4 C. tropicalis and 2 C. krusei. In our study two isolates of C. krusei were sensitive to ketoconazole, clotrimazole and miconazole. In addition both isolates were resistant to fluconazole, nystatin, econazole and terbinafine. Dose dependent was only observed about itraconazole. Only one isolate of C. tropicalis was sensitive to miconazole and terbinafine and two isolates were sensitive to clotrimazole (Table 2).
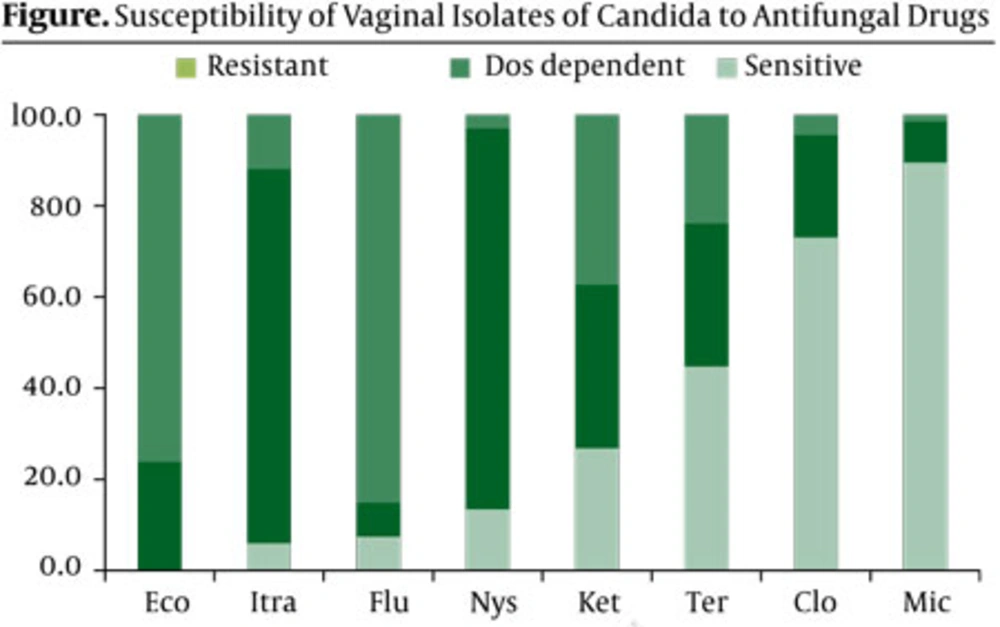
All eight isolates of C. glabrata were resistant to fluconazole, whereas all isolates were highly sensitive to miconazole. Highest sensitivity of C. albicans to antifungal drugs was seen against miconazole (49 of 53 isolates) followed by, clotrimazole (41 of 53), terbinafine (28 of 53) and ketoconazole (13 of 53). 43 of 53 isolates were also resistant to fluconazole and econazole antifungals (Table 2). In the present study, majority of isolates (85.1%) were resistant to fluconazole followed by 76.1% resistance to econazole (Figure). On the other hand, the best choice for the treatment of isolates was miconazole followed by clotrimazole, terbinafine and ketoconazole.
| Nystatin | ≥ 25 | 17-24 | ≥ 16 |
| Fluconazole | ≥ 19 | 15-18 | ≥ 14 |
| Ketoconazole | ≥ 30 | 23-29 | ≤ 22 |
| Clotrimazole | ≥ 20 | 12-19 | ≤ 11 |
| Miconazole | ≥ 20 | 12-19 | ≤ 11 |
| Itraconazole | > 16 | 10-15 | < 9 |
| Econazole | ≥ 30 | 23-29 | ≤ 22 |
| Terbinafine | ≥ 20 | 12-19 | ≤ 11 |
Criteria of Susceptibility and Resistance of Antifungal Disks
| Itraconazole | 4 | 41 | 8 | 0 | 8 | 0 | 0 | 4 | 0 | 0 | 2 | 0 | 4 (6.0%) | 55 (82.1%) | 8 (11.9%) |
| Fluconazole | 5 | 5 | 43 | 0 | 0 | 8 | 0 | 0 | 4 | 0 | 0 | 2 | 5 (7.5%) | 5 (7.5%) | 57 (85.1%) |
| Ketoconazole | 13 | 17 | 23 | 3 | 4 | 1 | 0 | 3 | 1 | 2 | 0 | 0 | 18 (26.9%) | 24 (35.8%) | 25 (37.3%) |
| Terbinafine | 28 | 18 | 7 | 1 | 3 | 4 | 1 | 0 | 3 | 0 | 0 | 2 | 30 (44.8%) | 21 (31.3%) | 16 (23.9%) |
| Clotrimazole | 41 | 11 | 1 | 4 | 4 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 49 (73.1%) | 15 (22.4%) | 3 (4.5%) |
| Miconazole | 49 | 4 | 0 | 8 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 60 (89.6%) | 6 (9.0%) | 1 (1.5%) |
| Nystatin | 7 | 46 | 0 | 2 | 6 | 0 | 0 | 4 | 0 | 0 | 0 | 2 | 9 (13.4%) | 56 (83.6%) | 2 (3.0%) |
| Econazole | 0 | 10 | 43 | 0 | 4 | 4 | 0 | 2 | 2 | 0 | 0 | 2 | 0 (0.0%) | 16 (23.9%) | 51 (76.1%) |
Sensitivity of Tested Isolates Against Several Antifungal Drugs
5. Discussion
Vulvovaginal candidiasis is one of the most common opportunistic fungal infections among adult women during their lifetime. Several researches have shown that the infection increases during two to three last decades. Clotrimazole and fluconazole are the two antifungal drugs that are widely used in the treatment of vulvovaginal candidiasis. The main agent of vulvovaginal candidiasis is C. albicans; however, it seems non-albicans species (C. krusei and C. glabrata) of Candida appear to be increasing (20). C. glabrata is the second commonest agent in vaginal infections in most regions (20). The sensitivity patterns of Candida isolates varies among studies in different countries (21, 22) unfortunately it is shown that resistance to antifungal azoles has been increased in recent years (21). In addition, sources of Candida were also affecting on sensitivity to antifungal drugs (17, 21).
Pelletier et al., believed that clotrimazole resistance was present in HIV-infected isolated of C. albicans (23). In Jordan, for example, Al-Abeid et al. showed that all tested Candida were susceptible to nystatin, miconazole, ketoconazole and fluconazole and C. albicans isolates were more susceptible to azoles than was C. glabrata (21). In addition, only 6% of C. albicans isolates were resistant to fluconazole. Quindos et al. study showed that 90.2% and 91.4% of isolates of Candida species were sensitive to fluconazole and ketoconazole, respectively (22). Whereas, our results show that 85.1% and 76.1% of tested isolates were resistant to fluconazole and econazole, correspondingly (Figure). Also our study shows that 100% of non-albicans species, were resistant to fluconazole, 64.3% to terbinafine and 57.1% to econazole. Most of non-albicans Candida species have higher azoles MICs than albicans species (1).
Terbinafine is a synthetic antifungal drug with fungicidal activity against dermatophytes, moulds and fungistatic activity against Candida species (24). Several researches have shown that terbinafine is the first choice for the treatment of dermatophytosis (25, 26), however, few details are available about its effects on vaginal isolates of Candida. Despite the fact our results show that terbinafine is effective against 76.1% of vaginal isolates. Therefore, terbinafine therapy can be considered as a good therapeutic option in the management of vaginal candidiasis. In conclusion antifungal sensitivity testing reveals that vaginal isolates of Candida were most sensitive to miconazole, clotrimazole, and terbinafine, and least sensitive to econazole, followed by fluconazole and ketoconazole.