1. Background
Aspergillus species are a common group of filamentous fungi, which have universal dissemination and are readily recovered from; soil, decaying vegetation, air and many other environments (1). They produce a large number of conidia (spores), which are turned into aerosols and distributed in the environment. Because of their small size (< 5 µm) and the aerodynamic properties of the Aspergillus conidia, they can pass through the upper respiratory tract defenses and may reach distal regions of the lungs (2). Aspergillus is a mould that is responsible for a gamut of respiratory diseases ranging from; saprobic colonization to rapidly invasive disseminated disease, especially in individuals with weakened immune systems (3). Aspergillus species are an important source of allergen proteins and have been shown to produce 90 different allergens (4). The clinical spectrum of Aspergillus associated hypersensitivity respiratory disorders includes; asthma, allergic bronchopulmonary aspergillosis (ABPA), allergic Aspergillus sinusitis (AAS) and hypersensitivity pneumonitis. Although A. fumigatus is the most common Aspergillus species known to trigger allergic disorders; A. niger, A. flavus, A. nidulans, A. oryzae and A. terreus have also occasionally been responsible for these conditions (5).
Tea (Camellia sinensis) is one of the most common drinks in the world, enjoyed by many people chiefly in the Far East, Middle East, Europe, Australia and Africa. It has been extensively cultivated in the north of Iran since ancient times and it is known to carry various mycoflora just like any other crop plants, some of which may secrete toxins. Previous studies have shown that the most common fungi isolated from tea gardens are; A. flavus and other species of Aspergillus and Penicillium (6). The processing of black tea involves a series of stages including; withering, rolling, fermenting and firing, before the final product is completed (7). Fungi have been isolated from all stages of tea processing and manufacturing, and recontamination of the final product after firing may also occur during sorting and packaging (8). The possibility that tea may serve as a vehicle for human pathogens has been reported in earlier studies (9). However, no systematic study has been carried out concerning the air spora of tea gardens in Iran, up to now. The high average temperatures and relative humidity of the Gilan and Mazandaran provinces, which are accompanied by poor conditions and prolonged duration of tea storage, promotes the growth of different fungi, in particular Aspergillus species.
2. Objectives
The purposes of this study were to isolate Aspergillus flora from tea gardens and factories air in certain regions of Gilan and Mazandaran provinces in northern Iran and to determine the protein patterns of various Aspergillus isolates.
3. Materials and Methods
3.1. Isolation and Identification of Aspergillus Species
Thirteen regions of Gilan and Mazandaran provinces were selected for data collection; Rasht, Lahijan, Amlash, Rudsar, Foman, Siahkal, Shaft, Langerud, Ramsar, Tonekabon, Chalus, Somesara and Astaneh. The samples were collected using five petri dishes including malt extract agar (MEA) (Merck Co., Darmstadt, Germany), yeast extract agar (YEA) (Merck Co., Darmstadt, Germany), Czapek Dox agar (CDA) (Sigma, St Louis MO, USA), Czapek yeast extract agar (CYEA) (Sigma, St Louis MO, USA) and Sabouraud dextrose agar (SDA) (Merck Co., Darmstadt, Germany) containing chloramphenicol (100 ppm) and tetracycline (50 ppm), from May to October of 2006 to 2008 (10). With the exception of rainy days, samples were exposed to the air for 30 min at a height of 1.5 m above the ground. After five days incubation at 25o C, the colonies were isolated in the various media and observed for the formation of macrocolonies. Micromorphology was observed after microcultivation and Aspergillus strains were identified using the classifications of Klich (11), Kojakivic (12) and Samson (13).
3.2. Cell Fractionation and Crude Extract Preparation
Each fungal isolate was cultured in Czapek yeast extract agar at a temperature of 25o C for 5 days and then incubated in Czapek Dox broth medium. Incubated broth media were kept at 25o C for 7 days on a gyratory shaker (200 rpm). Fungal mycelia and spores were separated from the broth media by centrifugation at 3 000 g for 15 min. The pellets were washed 3 times with 10 mM Phosphate buffered saline (PBS), pH 7.5 by centrifugation at 3 000 g for 15 min. The fungal elements were disrupted in liquid nitrogen containing glass beads (diameter; 1 mm) for 25 min. After cell disruption, the crude extracts were separated from other cell components by centrifugation at 3 000 g for 15 min. The extracts were sterilized using a filter (0.2 µ, Sartorious, USA) and stored at – 20o C until required.
3.3. Determination of Protein
Protein concentration of the sample was determined after reconstituting in 50 and 100 μl of distilled water by the Bradford method (14).
3.4. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was performed using the Laemmli method (15). The fungal extracts were dissolved in a sample buffer (Novex, San Diego, CA, USA) and boiled for 5 min. Aliquots of the sample (volum = 10 μl, which equals 60 μg of the protein) and standard markers (3 ± 185 kDa) (Novex, San Diego, CA, USA) were applied to a Novex precast NuBis-Tris gel (4 ± 12%) for the separation of fungal antigenic proteins. Electrophoresis was performed with a Novex X cell II mini-cell for 40 min at a constant voltage of 200 watt. The gel was fixed and stained with 0.1% Coomassie Brilliant Blue.
3.5. Statistical Analysis
The data analysis was undertaken with a SPSS (version no. 12) program using the Student’s t-test statistical methuod for analysis of significance. A p value less than 0.05 was regarded as significant.
4. Results
The results of the aeromycoflora study showed that the air of tea gardens and factories is never free of fungal spores. The sum of 4 157 (51.3%) and 3 950 (48.7%) Aspergillus colonies were obtained from the tea gardens and factories of Gilan and Mazandaran provinces, respectively. Of those colonies, 16 species were identified from gardens and 14 species from factories. As illustrated in Table 1, A. niveus (14.4%), A. unguis (12.6%) and A. alliaceus (9.7%) were the most frequent Aspergillus species isolated from the tea gardens, while A. melleus (14.3%), A. niger (12.3%) and A. flavus (11.6%) were found to be the most predominant Aspergillus species obtained from the tea factories. Our results showed that toxigenic species such as; A. flavus and A. parasiticus, were higher in number than the other Aspergillus species, especially inside the tea factories. Table 1 shows the population of different Aspergillus species from 11 tea gardens and 13 tea factories in a number of Iranian regions. The highest number of Aspergillus species that were obtained from tea gardens were associated with the regions of Lahijan (23.9%), followed by Rudsar (14.7%) and Rasht (14.3%). The tea factories with the highest level of Aspergillus species contamination were found in Rasht (18.8%), followed by Lahijan (16.4%) and Rudsar (11.8%).
| Tea Gardens | Tea Factories | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rasht | Rudsar | Lahijan | Foman | Ramsar | Shaft | Langerud | Chalus | Amlash | Siahkal | Tonekabon | Rasht | Rudsar | Lahijan | Foman | Ramsar | Shaft | Chalus | Langerud | Chalus | Amlash | Siahkal | Tonekabon | Somesara | Astaneh | |
| A. fumigatus | 150 | 14 | 55 | 0 | 31 | 0 | 0 | 6 | 28 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. fumigatus | 0 | 0 | 88 | 140 | 0 | 0 | 0 | 0 | 0 | 92 | 0 | 0 | 0 | 89 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 93 | 86 | 0 | 0 |
| A. unguis | 115 | 118 | 117 | 53 | 120 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 67 | 0 | 0 | 0 | 0 | 0 | 0 | 65 | 0 | 0 | 52 | 0 |
| A. terreus | 0 | 7 | 0 | 0 | 0 | 40 | 33 | 0 | 0 | 0 | 0 | 66 | 0 | 0 | 64 | 0 | 124 | 27 | 0 | 27 | 0 | 0 | 0 | 0 | 0 |
| A. niveus | 104 | 151 | 103 | 0 | 0 | 105 | 0 | 135 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. wentii | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 101 | 0 | 0 | 0 | 128 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 102 | 0 | 0 | 0 | 0 |
| A. flavus | 68 | 36 | 0 | 61 | 22 | 0 | 34 | 0 | 0 | 16 | 9 | 147 | 0 | 80 | 0 | 0 | 137 | 11 | 0 | 11 | 17 | 0 | 0 | 63 | 4 |
| A. sojae | 0 | 18 | 41 | 5 | 0 | 19 | 0 | 0 | 0 | 0 | 0 | 69 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 30 |
| A. parasiticus | 0 | 0 | 123 | 0 | 139 | 95 | 0 | 0 | 0 | 0 | 0 | 96 | 0 | 0 | 94 | 0 | 0 | 0 | 43 | 0 | 45 | 0 | 0 | 0 | 0 |
| A. alliaceus | 0 | 0 | 0 | 85 | 131 | 0 | 0 | 0 | 54 | 0 | 132 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. niger | 0 | 138 | 84 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 78 | 0 | 0 | 0 | 129 | 0 | 153 | 47 | 153 | 0 | 0 | 0 | 79 | 0 |
| A. awamori | 126 | 0 | 76 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 | 0 | 0 | 0 | 0 | 42 | 0 | 0 | 0 | 0 | 0 | 0 |
| A.carbonarius | 0 | 0 | 75 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 71 | 74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. foetidus | 0 | 0 | 0 | 83 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 77 | 0 | 46 | 0 | 39 | 0 | 0 | 0 | 0 |
| A. ochraceus | 32 | 0 | 107 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 70 | 125 | 106 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. ostianus | 0 | 130 | 26 | 37 | 0 | 0 | 0 | 0 | 0 | 0 | 136 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. melleus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 127 | 142 | 99 | 0 | 0 | 98 | 97 | 0 | 97 | 0 | 0 | 0 | 0 | 0 |
| A. candidus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 90 | 0 | 82 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 145 | 87 | 0 |
| Total | 595 | 612 | 995 | 464 | 443 | 262 | 67 | 141 | 183 | 108 | 287 | 743 | 466 | 646 | 158 | 253 | 312 | 260 | 205 | 260 | 268 | 93 | 231 | 281 | 34 |
Number of Aspergillus Species Isolated From Different Tea Gardens and Factories Air in Gilan and Mazandaran Provinces.
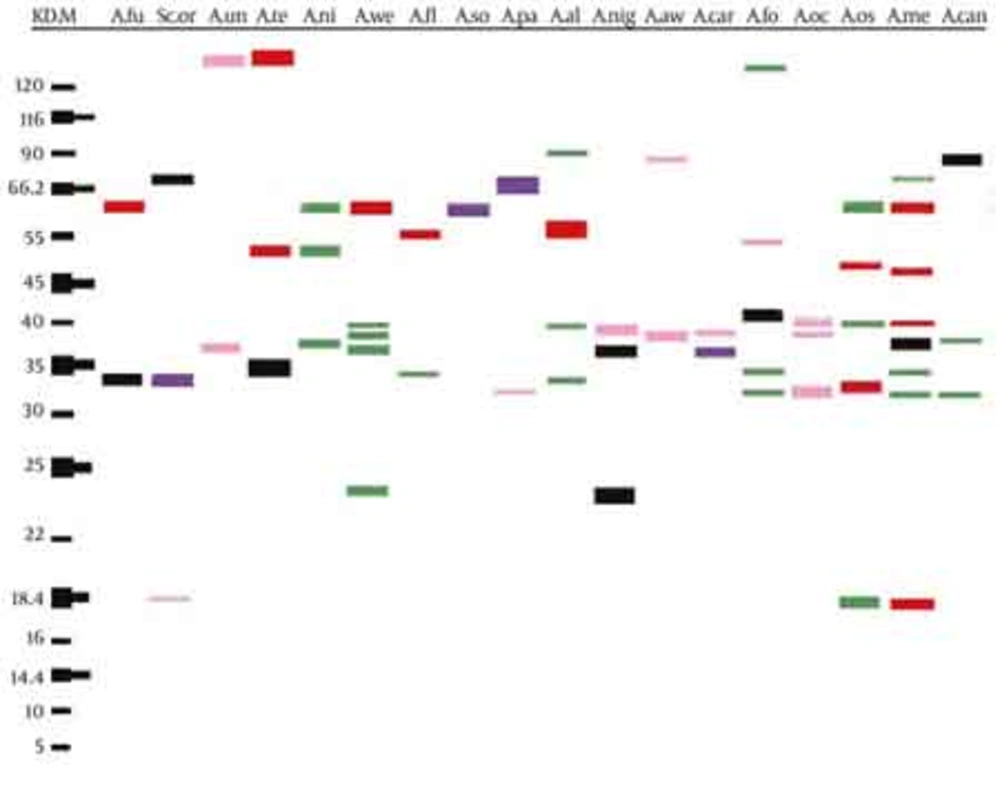
In the present study, results from the SDS-PAGE method indicated that extracts obtained from various Aspergillus species had 55 protein bands, which ranged from 5 to 120 kDa (Figure 1 and Table 2). Most bands were concentrated between 14.4 and 55 kDa in all of the species. Among these species, A. foetidus had the maximum number of protein bands (22 bands) ranging from 14.4 to 120 kDa and A. unguis had the minimum protein bands (5 bands) ranging from 30 to 120 kDa. Among the different bands, protein bands with a molecular weight of 120 kDa were present in all species of Aspergillus except for; A. fumigatus, A. parasiticus, A. carbonarius, A. ochraceus and A. ostianus. A band with a weight of 116 kDa was observed in all of the 18 species of Aspergillus except for; A. wentii, A. parasiticus and A. awamori. Thus, common protein bands including 120 and 116 kDa were detected with a frequency of 72.3% and 83.4% in different species of Aspergillus, respectively. Regarding the results of common protein bands and according to the statistical analysis, no meaningful relationship was observed among the protein bands obtained from different Aspergillus species in this current study.
| Protein bands, kDa | Total (bands) | |
|---|---|---|
| A. fumigatus | 116, 66.2, 45, 35, 30, 16.4 | 6 |
| Sclerocleista ornata | 120, 116, 110, 68, 60, 50, 45, 35, 26, 18.4, 16 | 11 |
| A. unguis | 120, 116, 55, 37, 30 | 5 |
| A. terreus | 120, 116, 66.2, 60, 55, 45, 35, 30, 27 | 9 |
| A. niveus | 120, 116, 70, 66, 55, 38 | 6 |
| A. wentii | 120, 66.2, 47, 45, 40, 37, 35, 28, 25, 24, 14.4 | 11 |
| A. flavus | 120, 116, 90, 66.2, 59, 55, 45, 44, 40, 35, 32, 27, 26, 22, 18.4, 17.6, 14.4 | 17 |
| A. sojae | 120, 116, 70, 66.2, 40, 30, 28, 14.4 | 8 |
| A. parasiticus | 116, 66.2, 70, 55, 50, 45, 42, 37, 33, 25, 17, 15, 14.4, 10, 5 | 15 |
| A. alliaceus | 120, 116, 92, 57, 47, 40, 35, 34, 30, 14.4 | 10 |
| A. niger | 120, 116, 75, 57, 45, 40, 37, 35, 32, 25, 24, 14.4 | 12 |
| A. awamori | 120, 90, 45, 40, 25, 24, 20, 18.4 | 8 |
| A. carbonarius | 116, 90, 66.2, 55, 54, 45, 43, 39, 37, 34, 33, 30, 27, 25, 23, 18.4, 17.4, 14.4, 10 | 19 |
| A. foetidus | 120, 116, 95, 90, 66.2, 55, 45, 44, 43, 42, 41, 37, 35, 33, 30, 25, 24, 20, 10, 18.4, 17.4, 14.4 | 22 |
| A. ochraceus | 116, 66.2, 47, 45, 44, 41, 40, 39, 34, 30, 29, 24, 20, 19.4, 18.4, 17.4, 15.4, 14.4, 10 | 19 |
| A. ostianus | 116, 66.2, 55, 44, 43, 41, 37, 32, 30, 27, 18.4, 16.4, 10 | 13 |
| A. melleus | 120, 116.2, 90, 80,70, 66.2, 53, 46, 42, 40, 38, 35, 33, 30, 20.4, 18.4, 17, 14.4 | 18 |
| A. candidus | 120, 116, 90, 55, 45, 40, 38, 33, 26 | 9 |
Number of Protein Bands Observed by the SDS-PAGE Technique in Each of the 18 Aspergillus Species Isolates.
Abbreviations: M, Marker; A.fu, Aspergillus fumigatus; Sc.or, Sclerocleista ornata; A.un, Aspergillus unguis; A.te, Aspergillus terreus; A.ni, Aspergillus niveus; A.we, Aspergillus wentii; A.fl, Aspergillus flavus; A.so, Aspergillus sojae; A.pa, Aspergillus parasiticus; A.al, Aspergillus alliaceus; A.nig, Aspergillus niger; A.aw, Aspergillus awamori; A.car, Aspergillus carbonarius; A.fo, Aspergillus foetidus; A.oc, Aspergillus ochraceus; A.os, Aspergillus ostianus; A.me, Aspergillus melleus; A.can, Aspergillus candidus
5. Discussion
Aspergillus species are well-known as causative agents of opportunistic infections in humans. Aspergillus antigens can also induce the development of hypersensitive respiratory processes (4), although their frequency, epidemiological characteristics and clinical relevance are still unknown, this is partly due to insufficient knowledge of these antigens.
In this present study, a total of 4 157 and 3 950 Aspergillus colonies were obtained from tea gardens and factories, respectively, from different regions of Gilan and Mazandaran provinces. The most frequent Aspergillus species isolated from the tea gardens and factories were found to be A. niveus (14.4%) and A. melleus (14.3%), respectively. In a study conducted by Sharma (16), A. fumigatus had the highest percentage contribution (18%), followed by A. flavus (12.6%), A. niger (9.2%), A. ochraceus (2.3%) and A. japonica (2.3%) in the air of the Darjeeling tea garden, India. Hasan and Abdel-Sater (17) indicated that Aspergillus was the most predominant genus encountered in all of the black tea samples comprising 92.3% of the total fungi. From the Aspergillus genus, 11 species and 1 variety were identified of which; A. flavus, A. fumigatus, A. niger and A. terreus were the most common. Other studies have reported Aspergillus species to be the most dominant fungi in all of the tea brands (18, 19).
A few of the fungal isolates are known to be mycotoxin producers, such as; A. flavus and A. parasiticus (20). In this study, the occurrence of these toxigenic fungi was high in comparison to that of the other Aspergillus species, especially in the tea factories. Our results are not in agreement with Dutta et al. (21), who reported a low frequency of aflatoxigenic Aspergillus species in the air of a tea factory in the Cachar district, Assam, India. Although not all A. flavus strains are aflatoxigenic, a high incidence of aflatoxigenic strains (50-100%) are usually found among A. flavus isolates (22). Keeping the above view in mind, information regarding the probable mycotoxin producing air mycoflora in relation to the tea garden and factory and their mycotoxin producing ability in tea, it should be given due importance. Mycotoxins are secondary fungal metabolites with well-known health effects including; carcinogenesis, immunosuppression and cytotoxicity on alveolar macrophages (23). They are products that become poisonous and elicit a toxic response known as mycotoxicoses when food or feed containing them is eaten by humans or animals (24). There is evidence that aflatoxins can become air-borne and circumstantial evidence suggests that aflatoxins may also occur in the air. In fact, aflatoxins have been found in; spores, fungal hyphae and substrate materials, all of which could be released into the air when toxigenic fungi sporulate on indoor building materials. Their rate of inhalation can be up to 40 times greater than by ingestion (25). This raises the possibility of potentially hazardous exposures to the inhabitants of contaminated spaces.
In the present study, the highest number of Aspergillus species obtained from the tea gardens and factories were associated with the regions of Lahijan (23.9%) and Rasht (18.8%), respectively. Aerosolized Aspergillus spores are found nearly everywhere so we are routinely and almost constantly exposed to them. They can cause human and animal diseases through the production of mycotoxins, by the induction of allergenic responses and through localized or systemic infections (26). Occupational mycoses are forms of extrinsic allergic alveolitis. They are all inflammatory reactions caused by breathing in high concentrations of fungal spores and other antigenic organic matter. Farmer’s lung, one of the best known of these, is an occupational mycosis correlated with inhalation of high concentrations of Aspergillus spores from contaminated agricultural products (27).
The results from the SDS-PAGE analysis indicated that extracts obtained from various Aspergillus species contained 55 protein bands with molecular weights between 5 and 120 kDa. The highest protein bands were associated with A. foetidus (22 bands) in a range from 14.4 to 120 kDa, while the lowest ones were detected in A. unguis (5 bands) in a range from 30 to 120 kDa. Protein bands of 120 and 116 kDa had 72.3% and 83.4% frequencies in different species of Aspergillus, respectively. Our results are in close agreement with previous investigations. In a study by Saeednejad et al. (28), 69 protein bands with molecular weights between 11.5 and 178 kDa were identified. Among these , protein bands with molecular weights of 15, 23.5, 27, 33.5 and 61 kDa were found in two species, A. fumigatus and A. flavus, protein bands 28.5, 40 and 47 kDa were found in two species of A. flavus and A. niger, and a band with 120 kDa was found in A. fumigatus and A. niger. Identification of a protein in a complex antigenic extract on the sole basis of its molecular weight is a difficult process and sometimes the same antigen may have small differences in molecular weight in different studies. In addition, the differences observed in molecular weight may be related to differences in the calculation of the molecular weight or to the presence of several antigens with the same molecular weight, the species used as the source of antigenic proteins and sometimes even the methods of detection.
In conclusion, A. niveus and A. melleus were the predominant Aspergillus species found in tea gardens and factories air, respectively. In SDS-PAGE, 22 and 5 protein bands were detected in A. foetidus and A. unguis, respectively. The whole-cell proteins profile obtained by the SDS-PAGE analysis can provide valuable criteria for serological and immunological studies of tea workers exposed to occupational aeroallergens. Also, the results obtained do not support the concept that such protein patterns can be used in the identification of species, however, it suggests that this technique might be of value in assessing the degree of similarity among species of the same genus or of closely related genera.