1. Background
Cervical cancer is the second common women malignancy all over the world. Although routine screening of suspected cases by Pap smear has reduced the cervical cancer mortality rate, reports from over the world indicate that still 500,000 new cases of progressive cervical cancer are emerging annually (1). Human papillomavirus (HPV) is a double-stranded DNA virus and a member of Papillomaviridae family. About 118 species of this family have been known till now. Members of 5 genus of this family including alpha papillomavirus harbor some of human organelles. Forty types of alphapa pillomavirus infect human female genital tracts. Some members of this genus including genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 59 and 66 are responsible for human cervical, mucosal anogenital, head and neck cancers (1, 2).
Persistent HPV infection might lead to cervical intraepithelial neoplasia (CIN 2, 3) and Adenocarcinoma In-Situ (AIS), and in case of untreated, would increase the cancer risk (3). Regular gynecological screening and swift clinical diagnosis of precancerous lesions are significant preventive measurements of cervical cancer (1). Permanent expression of HPV E6 and E7 in human cell lines, stimulates the transformation of human cells. Expression of these viral proteins is required to ensure the proliferative stage of infected cells. These proteins can deregulate the host cell growth cycle by binding and inactivating tumor suppressor proteins, cell cyclins, and cyclin-dependent kinases (4).
Currently the most common screening test used for cervical cancer is Pap smear. In this test the cytomorphology of the cervical cells are evaluated and the degree of abnormal cells are determined and graded. Pap smear is a highly specific and screening test through this test the incidence of cervical cancer following detection has been lowered. Sampling process, spreading of the cells on microscopic slides, fixation of the slides, staining of the smears and pathologist expertise are interfered in the specificity of the Pap smear (1, 5). On the other hand, the Pap smear sensitivity is low (25-50%) so that false negative results are common (6, 7). Reports show that some of the false negative results related to progressing cervical neoplasia (CIN) (8). PCR test to detect HPV DNA is highly sensitive (6) and has been recommended for detection of CIN in parallel to cytological tests, and it has been shown that PCR analysis of Pap smear considerably improved the chances of CIN 2,3 detection (8, 9).
2. Objectives
According to the important HPV role on cervical cancer incidence, and recommendation of HPV detection by in combination of Pap smear for the mentioned purposes, the aim of this study was to detect and genotype HPV strains obtained from cervical tissues. Consequently, the results of present study might help physicians to prognosis and manage cervicalneoplasia and abnormalities. Also it can fill the gaps on epidemiological data about HPV genotypes distribution in Iran.
3. Materials and Methods
3.1. Sample Collection
Sixty paraffin-embedded collected cervical biopsy samples from the patients with warts, protruded tissue mass, skin scars, or pain. These criteria were used for sampling from the patients referred to the pathology department of Ahvaz Imam Khomeini hospital from January 2009 to December 2010. The other considered citeria were the age of the patients (>15 years old) and abnormal histological report. The blocks of biopsy samples were transferred to virology laboratory of university.
3.2. Preparation of Biopsies to Extract the DNA
3 sections from each block were prepared. The diameter of each section was 10µm. The biopsy sections were placed into 1.5 mL DNase free microtubes. For deparaffinization, 1 mL xylene was added in each tube and the tubes were incubated at 45 °C. After 15 minutes the xylene was removed. This step was performed two times. To completely remove the remaining amount of xylene, the tissue was washed with ethanol 90% and then ethanol 70%. The tubes were kept at room temperature with opened cap to completely evaporate ethanol.
3.3. DNA Extraction
DNA was extracted from deparaffinized biopsy sections by DNP kit (Cinnagen, Iran). Each sample was treated with a buffer supplied by kit and Proteinase K and incubated at 55°C for 3 hours, the lysis buffer was added and vortexed for 20 seconds. At this step the tissue was completely digested and a clear solution was obtained. Then precipitation solution of the kit was added to the tube and the tube was centrifuged for 10 minutes at 12000 rpm. The pellet s washed twice with ethanol. Ethanol was removed and the tubes were stand at room temperature uncapped for about 5 minutes to evaporate ethanol completely. DNA pellet was dissolved in 50 µL DNase-free high-quality grade water (Millipore, USA). DNA was saved as template of PCR test at -20°C.
3.4. PCR
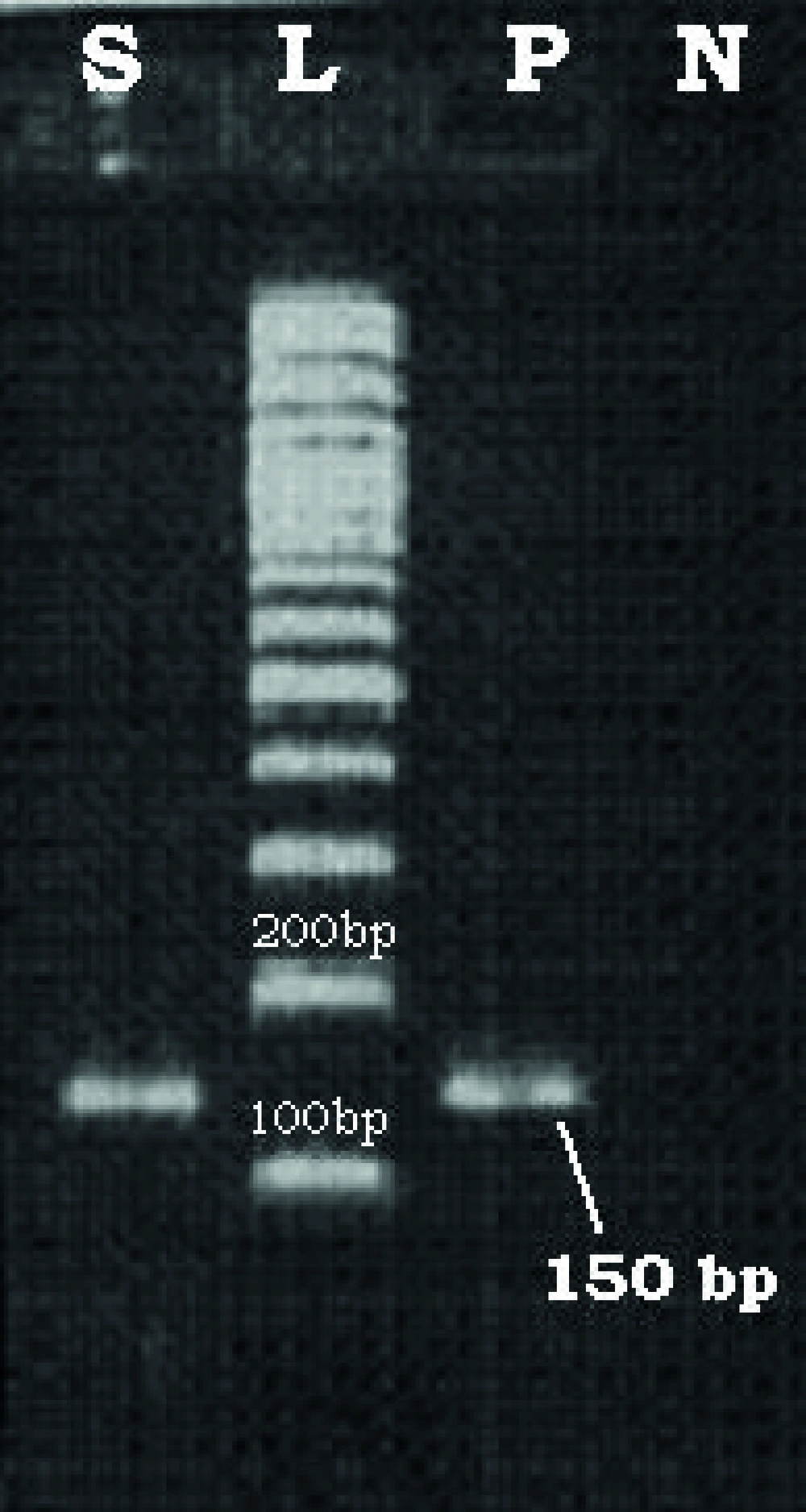
At first, the absence of PCR inhibitors was evaluated using β-Globin primers (10). Then HPV DNA PCR reaction was prepared in 50 µL volume (11). The PCR reaction contained 5µL DNA template, 1µL dNTPs (10mM), 0.3µL Taq DNA polymerase (5U/µL), 5µL 10X PCR buffer, 1µ of each GP5+ and GP6+ primers (30 pmol), and 36.7 µL DNase free UHQ. PCR components were purchased from Roche (Germany). Themocycler (TC-512, Techne, UK) was programmed as follow: Initial denaturation, at 95°C for 5 minutes, then 35 cycles of: denaturation Tm, 95°C for 40 seconds, annealing Tm, 40°C for 40 seconds, and extension Tm was 72°C for 45 seconds. The final extension cycle was 72°C for 3 minutes. Six µL of PCR products were loaded on the 1.5% agarose gel and electrophoresed for about 1 hour at 100 volts. Following ethydium bromide staining the gel was visualized on UV Transiluminater
(Vilber Lourmat, France). A DNA band with 150bp was considered as a positive sample and subjected to genotyping. In all PCR tests a positive and negative control was included. One sample (obtained from Dr. Keyvani, Keyvan virology lab, Tehran, Iran) that previously determined positive for HPV DNA was used as positive control and DNA extraction product of a liver biopsy sample was used as negative control.
3.5. Genotyping
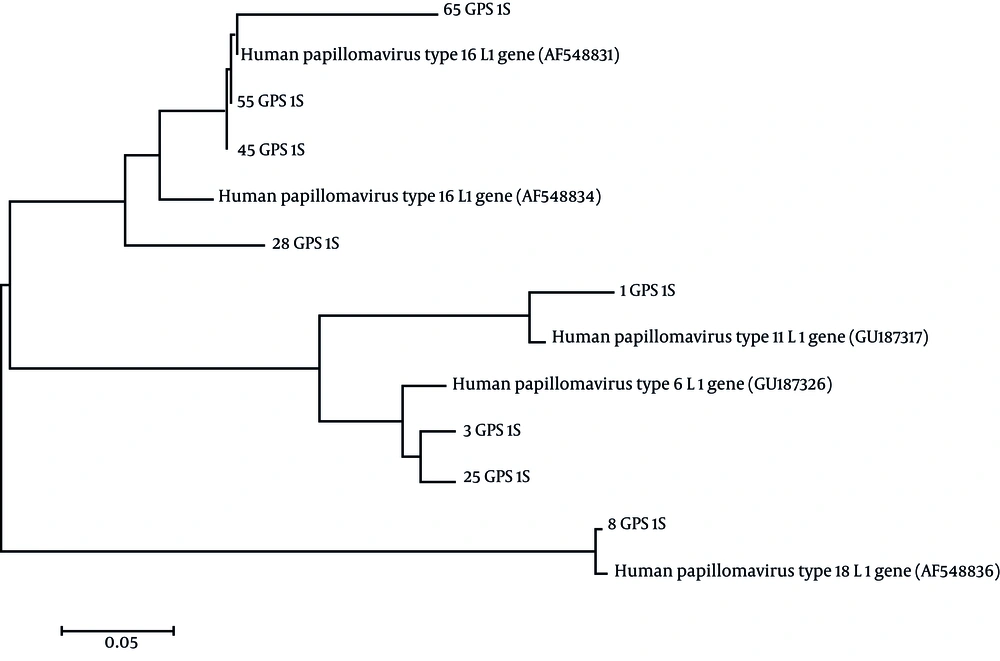
The genotype of HPV PCR positive samples were determined based on evaluation of PCR product sequences. PCR product of HPV DNA positive samples were separated by agarose gel electrophoresis and purified using the High Pure PCR Product Purification Kit (Roche, Germany). Purified products were sequenced by Bioneer Company (South Korea). Nucleotide sequences were corrected by visual inspection. Sequences were compared to HPV sequences available at Gene bank database at http://ncbi.nlm.nih.gov/ using BLAST. Moreover, to evaluate the genetic distances and phylogenetic tree, the sequences were aligned with Clustal W program using Molecular Evolutionary Genetics Analysis (MEGA) software version 5.0 (12) followed by construction of phylogenetic tree based on the Neighbor-Joining (NJ) algorithm (13). Finally, HPV genotypes were identified using BLAST and phylogenetic tree data.
4. Results
4.1. PCR
Sixty biopsy samples from the archive of pathology department, Ahvaz Imam Khomeini hospital were analyzed. The mean age of patients was 45 years (age range of 25-81 years old). Out of 60 samples, 8 specimens (13.3%) were positive for HPV DNA. Figure 1 shows the PCR results of HPV DNA positive samples.
4.2. Genotyping
After multiple sequence alignment, the phylogenetic tree was constructed (Figure 2) using molecular evolutionary genetics analysis (MEGA) software version 5.0 (13). Based on BLAST and phylogenetic tree information, the HPV genotypes were determined. Four HPV DNA positive samples (50%) were identified as genotype 16, 2 (25%) were determined as genotype 6, 1 positive sample (12.5%) was detected as genotype 18 and 1positive sample (12.5%) was identified as genotype 11.
5. Discussion
Cervical carcinoma is the second most prevalent female cancer worldwide (1). The survive of thousands of patients would be possible by rapid detection of this cancer. Positive HPV PCR is considered as a prognostic indicator of HPV cervical cancer and combination of PCR and cytological test have already applied for rapid diagnosis of CIN 2, 3 in the early-stage of the disease (6-10, 14). Since HPV is not routinely cultivable, the best techniques for detection of HPV are through molecular techniques such as PCR and genotyping. For above mentioned reasons we have decided to detect and genotype the HPV obtained from the cervical biopsy specimens. Besides, there is no information about predominant genotype of HPV among cervical biopsy samples in Khouzestan.
In this study, following PCR of the cervical biopsy samples and genotyping of the products, the result showed that the predominant genotype of HPV in Iran (Khouzestan) is HPV-16. This result is accordance to other studies performed in other parts of the world (7, 15-17). When HPV infection was determined by specific probes of 16 and 18 genotypes (high risk HPVs), both genotypes were reported as dominant HPVs(18, 19). The prevalence of HPV in cases with abnormal Pap smears or squamous cell carcinoma (SCC) was 24-67% (15-18). These records were reported 5.5-35% in cases with normal Pap smear results (7, 15, 17, 18, 20).
Similar screening studies performed to assess the HPV prevalence in normal Pap smears or normal biopsy samples in Iran,, demonstrated that 5.5-9% of the specimens were HPV positive (7, 18). The HPV prevalence in the present study was 13.3%.This amount was lower compared with the reported statistics of HPV in other parts of the world (15, 17, 20). Since detection of HPV is a more sensitive technique than cytomorphology and false negative results of the previous technique is overcome, we suggest detection of HPV along with cytomorphological test to this purpose. It could be a useful prognostic indicator in early-stage of and cervical cancer.