1. Background
Staphylococcus aureus is a hospital and community-acquired pathogen that causes a broad spectrum of diseases, ranging from skin and soft tissue infections to endocarditis and fatal pneumonia. This pathogenicity is associated with different enzymes and toxins such as enterotoxins, exfoliative toxin, toxic shock syndrome toxin, and Panton-Valentine leucocidin (PVL) (1, 2).
This bacterium has the remarkable ability to adapt to different antibiotics and now with the emergence of multi-drug resistant (MDR) bacteria, S. aureus is a warning for public health (1, 3). Methicillin-resistant S. aureus (MRSA) strains are able to grow in the presence of methicillin, oxacillin and nafcillin (4).
Methicillin resistance in S. aureus was initially detected in Europe in the 1960s, only one year after the introduction of methicillin (5, 6). Today, MRSA isolates are found not only in the hospitals of most countries but also in communities and are often resistant to several antibiotics (7). Clinical infections are most common in patients in hospital intensive care units, nursing homes, and other chronic care facilities; however, MRSAs are emerging as an important community acquired pathogen as well. Although there are some reports on the prevalence of vancomycin resistant S. aureus (VRSA) and vancomycin intermediate S. aureus (VISA), most MRSA isolates are susceptible to vancomycin and teicoplanin; therefore resistance increase to these antibiotics results in the limitation of treatment options and also the requirement of a new class of antibiotics (8, 9).
S. aureus is the most important cause of infection among hospitalized patients and also the second cause of infection among outpatients (10). Today, S. aureus is the leading cause of nosocomial pneumonia and the second leading cause of bloodstream infections in the world (11). MRSA is also dominant in intensive care unit (ICU) of hospitals in most parts of the world (12).
The emergence of novel community-acquired MRSA (CA-MRSA) strains has complicated the control and prevention of infections caused by MRSA isolates. The genetic and phenotypic properties of these CA-MRSA isolates are completely different from hospital acquired MRSA (HA-MRSA) isolates (13, 14). These differences in the structure of CA and HA-MRSA strains results in antibiotic resistance patterns differences of isolates influencing the hospital and community environments (15). Increasing rate of CA-MRSA isolates recovered from patients in different parts of the world, suggests that these strains are displacing among nosocomial MRSA strains and might behave similar to traditional nosocomial MRSA strains with respect to mortality and other various clinical outcomes (13, 16).
2. Objectives
There are several reports focusing on MRSA isolates prevalence in different parts of Iran. But, the current study aimed to analyze the prevalence and also antibiotic susceptibility patterns of MRSA and methicillin sensitive S. aureus (MSSA) isolated from three referral hospitals in Tehran.
3. Materials and Methods
3.1. Sample Collection
A total of 726 isolates of S. aureus were collected during 2007 to 2011 from three referral hospitals in different parts of Tehran including three university hospitals (A-C) in south, north and center of Tehran with the associated number of isolates 194, 321 and 211 respectively.
3.2. Isolation and Phenotyping of S. aureus
All of the isolates were confirmed as Staphylococcus genus by different biochemical tests such as Gram staining, catalase and oxidase (17). Catalase, Gram positive and oxidase negative isolates were defined as Staphylococcus. Isolates indicating staphylococcus characteristics were further analyzed by fermentation in manitol salt agar medium, DNase and coagulase tests (17). All S. aureus were DNase and coagulase positive and fermented manitol. S. aureus ATCC 29213 and S. epidermidis ATCC 35984 were used as negative and positive controls.
3.3. Antimicrobial Susceptibility Testing
Susceptibility of S. aureus isolates to oxacillin (1 µg), Kanamycin (30 µg), amikacin (30 µg), penicillin (5 µg), fusidic acid (10 µg), minocycline (30 µg), erythromycin (15 µg), clindamycin (2 µg), tobramycin (10 µg), rifampicin (2 µg), nitrofurantoin (50 µg), sulphamethoxazole-trimethoprime (1.25-23.75 µg), linezolid (10 µg), synercid (15 µg), chloramphenicol (30 µg), ciprofloxacin (30 µg), gentamicin (10 µg) and tetracycline (30 µg) (MAST Group, Merseyside, United Kingdom) was determined by disc diffusion method according to the guidelines of Clinical and Laboratory Standards Institute (CLSI) (18). All methicillin resistant strains were collected and MICs of oxacillin and vancomycin among MRSA isolates were determined by Etest (AB, Biomerieux, Marcy l'Etoile, France) according to the manufacturer's instructions and were repeatedby CLSI guidelines (19).
3.4. DNA Extraction
DNA extraction was done by High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany) according to manufacturer`s instructions with some modifications. The concentrations of all extracted DNAs were determined by Nanodrop 1000 (NanoDrop, Wilmington, USA). One micro liter of each DNA was used as template in PCR reaction.
3.5. PCR
PCR primers specific for mecA gene (mecA1: GTAGAAATGACTGAACGTCCGATAA and mecA2: CCAATTCCACATTGTTTCGGTCTAA) were selected from Jonas et al. (20) and synthesized by Tib-Molbiol (Berlin, Germany). The PCR mixture containing; 10X PCR buffer, taq DNA polymerase (0.5 U/μl) (HT Biotechnology, Cambridge, United Kingdom), each primer (1.6 μM), MgCl2 (1.2 μM) and each dNTP (0.64 μM). The PCR cycles for the isolates were as follows: an initial denaturation at 94°C for 5 min, with 30 cycles of denaturation at 94°C for 15s, annealing at 61°C for 15s and elongation at 72°C for 30s and final extension at 72°C for 5 min (20). PCR products were electrophoresed on a 1.5% agarose gel in a 0.5X Tris-Borate-EDTA (TBE) buffer and stained in ethidium bromide.
4. Results
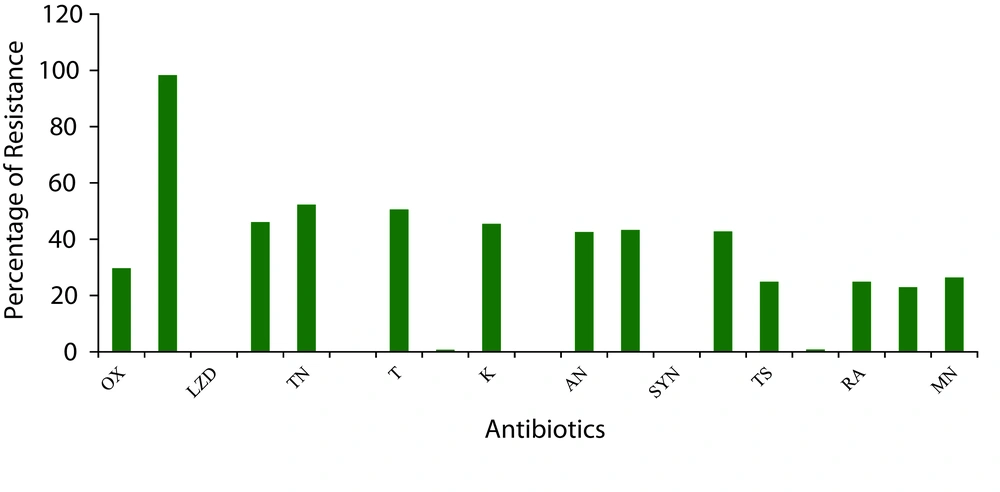
Totally, all suspected 726 isolates gathered from different hospitals were confirmed as S. aureus by standard biochemical tests. 216 isolates (29.7 %) were selected as MRSA and were analyzed more. As shown in Figure 1 , more than 98%, the highest level, of the S. aureus isolates were resistant to penicillin. All MRSA and MSSA isolates were susceptible to chloramphenicol, synercid and linezolid, and also more than 99% of the isolates were susceptible to nitrofurantoin and fusidic acid. Resistance to gentamicin, minocycline, SXT and rifampicin was less than 30%. Resistance to clindamycin, tobramycin and tetracycline was higher than other antibiotics and more than 40% of total isolates were resistant to these three antibiotics.
Abbreviations: OX, Oxacillin; P, Penicillin; LZD; Linezolid, CD; Clindamycin, TN; Tobramycin, C; Chloramphenicol; T, Tetracycline; NI, Nitrofurantoin; K, Kanamycin; VA,Vancomycin; AN, Amikacin; E, Erythromycin; SYN, Synercid; CIP, Ciprofloxacin; TS, Cotrimoxazole; FC, Fusidic Acid; RA, Rifampicin; GM, Gentamicin; MN, Minocycline
In antimicrobial susceptibility test of MRSA isolates, the number of bacteria resistant to penicillin, ciprofloxacin, tobramycin, kanamycin, erythromycin, clindamycin, tetracycline and amikacin were the highest ( Table 1 ). None of the isolates were resistant to chloramphenicol, synercid and linezolid. Only 6% of MRSA isolates were resistant to all antibiotics other than these three antibiotics. Seven percent of MRSA isolates were susceptible to all antibiotics except for penicillin.
| MRSA, No. (%) | MSSA, No. (%) | |
|---|---|---|
| Oxacillin | 216 (100) | 0 (0) |
| Penicillin | 216 (100) | 498 (98) |
| Clindamycin | 163 (75) | 172 (34) |
| Nitrofurantoin | 5 (2) | 0 (0) |
| Tobramycin | 196 (91) | 184 (36) |
| Tetracycline | 179 (83) | 189 (37) |
| Chloramphenicol | 0 (0) | 0 (0) |
| Fusidic Acid | 6 (3) | 0 (0) |
| Kanamycin | 184 (85) | 147 (29) |
| Amikacin | 181 (84) | 129 (25) |
| Vancomycin | 0 (0) | 0 (0) |
| Erythromycin | 201 (93) | 114 (22) |
| Ciprofloxacin | 205 (95) | 106 (21) |
| Synercid | 0 (0) | 0 (0) |
| SXT | 136 (63) | 45 (9) |
| Rifampicin | 147 (68) | 42 (8) |
| Gentamicin | 127 (59) | 40 (8) |
| Minocycline | 106 (49) | 86 (17) |
| Linezolid | 0 (0) | 0 (0) |
Frequency of Antibiotic Resistance of MRSA and MSSA Strains.
In the current study no vancomycin resistant MRSA strain could be isolated and the frequencies of vancomysin resistant S. aureus (VRSA) and vancomycin intermediate S. aureus (VISA) were zero.
According to the comparison of antibiotic resistance patterns among MRSA and MSSA isolates, as indicated in Table 1 , 98% of MSSA isolates were resistant to penicillin and it was the only antibiotic that most of the MSSA isolates were resistant to. In the case of clindamycin, tobramycin, tetracycline and amikasin more than 2.2 fold increase in resistance was observed among MRSA isolates in comparison to MSSA isolates. Moreover, in the case of amikacin this rate was increased to more than 3 fold. Interestingly, for erythromycin, ciprofloxacin, gentamicin and SXT this range varied from 4 to 7 fold. In MRSA isolates the rate of gentamicin resistance was 59%, while in MSSA isolates it was only 8%. Also for rifampicin, in MRSA isolates the rate of resistance was 68% but only 8% of MSSA isolates were resistant to this antibiotic. Seventy five percent of MRSA isolates were resistant to clyndamicin, while 34% of MSSA exhibited resistance to clindamycin. Not only MRSA isolates, but also MSSA isolates were susceptible to linezolid, synercid and chloramphenicol. Nitrofurantoin and fusidic acid were the two antibiotics that 98 and 97% of MRSA isolates exhibited susceptibility to them respectively, and also no resistance was observed among MSSA isolates to these antibiotics.
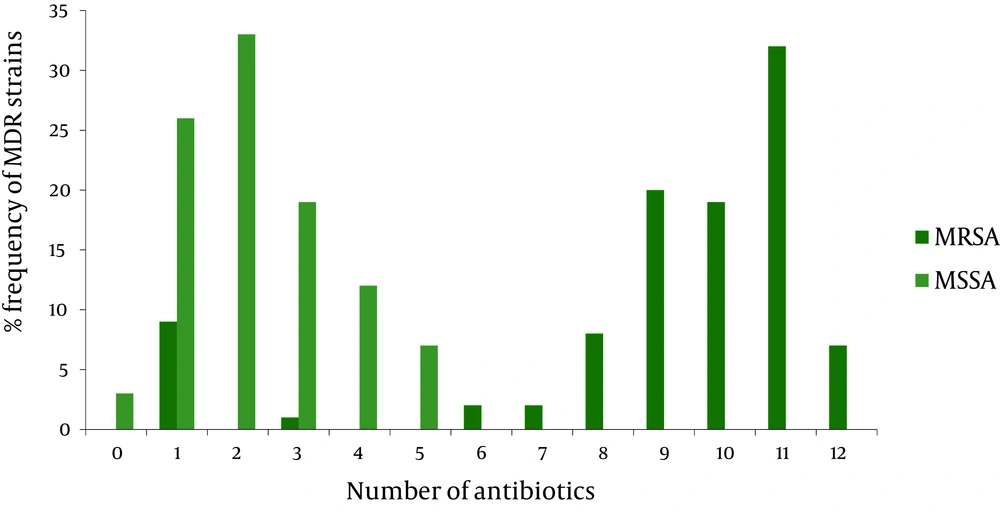
According to Centers for Disease Control (CDC), MDRs are defined as microorganisms, predominantly bacteria, that are resistant to one or more classes of antimicrobial agents (21). Therefore, in MRSA and MSSA isolates, 9 and 5 different multi drug resistance patterns were determined, respectively ( Figure 2 ). In the current study only 93% of MRSA isolates were resistant to at least three antibiotics, while 61% of MSSA isolates were resistant to at least two antibiotics. Here, only 6% of MRSA isolates were resistant to 12 antibiotics, and 34% were resistant to eleven antibiotics. In MSSA isolates, only 3% were susceptible to all antibiotics and 26% were resistant to one antibiotic.
In one hospital, the frequency of the MRSA isolates was completely different in comparison to other hospitals. In hospitals A-C, the frequency of MRSA isolates were 8, 9 and 32% respectively. The frequency of MRSAs isolated from different sources is shown in Table 2 . Thirty nine percent of MRSA isolates were from wounds. The lowest number of MRSA isolates was associated with ear and eye infections. The frequency of MRSA isolates were 19, 12, 10 and 8% in urine, sputum, CSF and nose cultures, respectively. Also, 5 and 5% of MRSA isolates were associated with blood and abscess cultures, respectively.
| Gynecology | Surgery | ENT* | ICU* | Respiratory | Ophthalmology | Pediatrics | Oncology | Urology | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Abcess | - | 1 | - | 2 | - | - | 4 | - | 4 | 11 |
| Blood | 2 | 3 | - | 1 | - | - | 2 | 3 | - | 11 |
| Ear | - | - | 3 | - | - | - | - | - | - | 3 |
| Eye | - | - | - | - | - | 2 | - | - | - | 2 |
| Nose | 5 | 2 | - | 3 | 3 | - | 4 | - | - | 17 |
| Sputum | - | - | 1 | 3 | 15 | - | 1 | 6 | - | 26 |
| CSF | 5 | 8 | - | 4 | - | - | - | 4 | - | 21 |
| Urine | 11 | - | - | 8 | - | - | 6 | 4 | 12 | 41 |
| Wound | 10 | 29 | - | 13 | - | - | 7 | 19 | 6 | 84 |
| Total | 33 | 43 | 4 | 34 | 18 | 2 | 24 | 36 | 22 | 216 |
Distribution of MRSAs Isolated From Different Samples and Different Wards.
Considerable differences were observed when the distributions of MRSA isolates in different wards were compared ( Table 2 ).Twenty , 15, 17, 16, 10, 11 and 8% of MRSA isolates were recovered from surgery and operation, gynecology, oncology, intensive care, urology, pediatrics and respiratory units respectively. Only 2 and 1% of the isolates were from ear, nose and throat (ENT), and ophthalmology unites, respectively.
Moreover, the frequency of MRSA isolates among elderly patients (61%) was higher than the others. Thirty four and 5% of MRSA isolates were associated with adults and children, respectively. Also, in contrast to women (41%), the frequency of methicillin resistance was the highest among S. aureus bacteria isolated from men (59%).
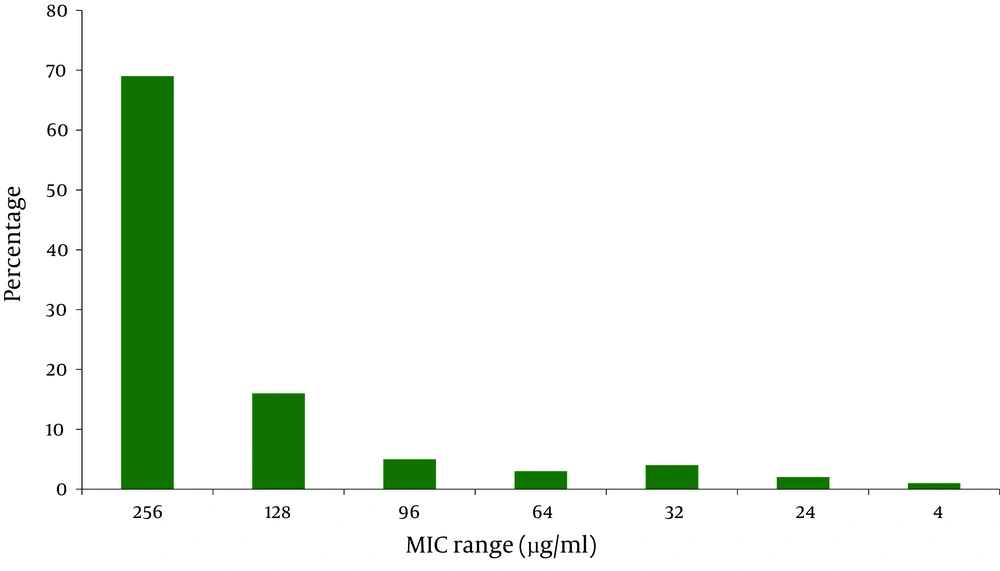
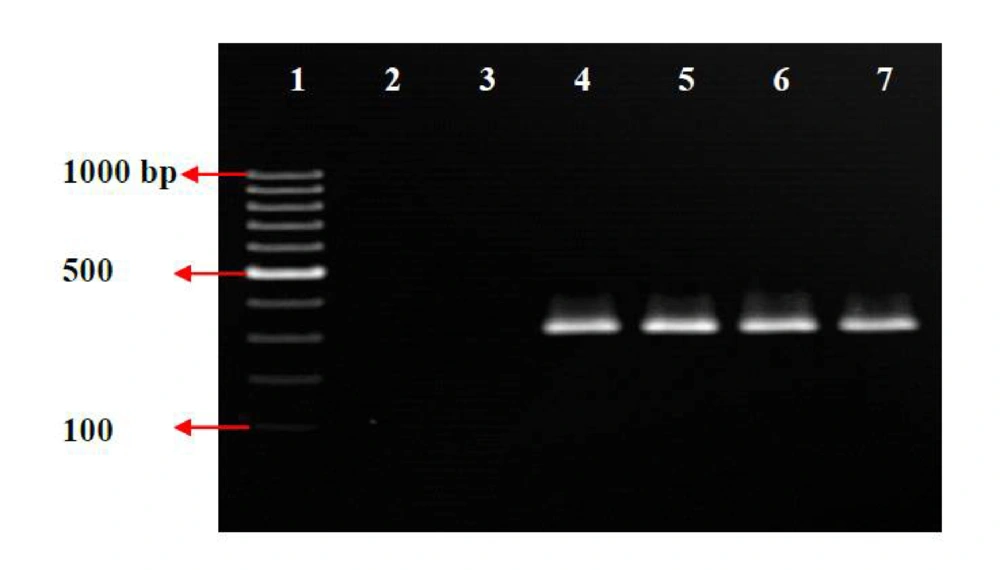
MIC results using Etest showed that 100% of MRSA isolates were resistant to oxacillin (MIC ≥ 4 µg/ml) ( Figure 3 ). Eighty five percent of MRSA isolates were highly resistant to oxacillin (MIC ≥ 128 µg/ml) and 4% of the isolates showed low resistance to oxacillin. The MIC ranges of 11% of MRSA isolates varied from 24 to 96 µg/mL. In PCR, mecA gene was detected in all 216 MRSA strains ( Figure 4 ) which confirmed all isolates as MRSA.
5. Discussion
Results of the current study indicated that the prevalence of MRSA isolates in three different hospitals in Tehran was 29.7%. This rate of resistance was lower than the other reports from Iran (42-90%) (8, 22-25). However, this variation of MRSA isolates in different geographical regions of Iran might also be due to several other factors like efficacy of infection control practices, healthcare facilities and antibiotic usage that vary from hospital to hospital. MRSA infection has recently become a serious problem in anti-microbial chemotherapy. During the past four decades, MRSAs have spread throughout the world and have become highly endemic in many geographical areas. It has been suggested that due to the changing pattern of antibiotic resistance in S. aureus, it would be wiser to have a periodical surveillance of these changes every 3 to 4 years (22, 23).
The current study indicated that resistance to linezolid, synercid and chloramphenicol was low and they were the most effective antibiotics against MRSA isolates. This may be due to low consumption of these antibiotics in Iran. This could, in turn, suggest the lack of horizontal transfer of resistant genes from other bacterial species to MRSA. Although chloramphenicol is a very effective antibiotic against MRSA isolates in vitro, its prescription is influenced by different side effects, a fact that could explain low resistance frequency (22, 23). Although Linezolid and synercid are the most effective antibiotics against MRSA isolates, their high cost limits their consumption for treatment purposes.
Vancomycin is the last resort and drug of choice to treat infections caused by MRSA isolates in the world, therefore the emergence of resistance to vancomycin could be an urgent warning for public health. Current results were inconsistent with other studies in Iran which have reported high prevalence (7%) of VRSA isolates (25). This might be due to using improper diagnostic methods. As recommended by CLSI, the standard methods for vancomycin resistance screening in S. aureus are, Etest, broth dilution and agar dilution; so the results of disk diffusion test is not reliable. Since, the incidence of nosocomial infections caused by MRSA isolates represents the measures for control and prevention in hospitals, the necessity to treat MRSA infections by vancomycin results in emergence of VRSA isolates. Therefore, this antibiotic should be prescribed with caution.
The pattern of antibiotic susceptibility of MSSA and MRSA isolates differed significantly. The MSSA isolates were susceptible to most of the antibiotics tested, although some resistance was observed to penicillin, tetracycline, tobramycin, and to some extent to clindamycin, amikacin and kanamycin, the antibiotics were often used to treat general infections. In contrast, in the case of MRSA, multiple drug resistance was common and only a few antibiotics were active against these isolates. MRSA strains were found to be more resistant to other antibiotics than MSSA strains. Significant difference (P value < 0.05) was observed in case of erythromycin, ciprofloxacin, tobramycin, tetracycline, gentamicin and amikacin. A significant difference was found between sensitivity patterns of MRSA and those of MSSA isolates (24, 26, 27).
Differences in the frequency of methicillin resistance among S. aureus bacteria isolated from various specimens might be due to prolonged antibiotic treatment of severely sick patients, which generally have longer hospital stays, resulting in enhanced selection pressure (24). Distributions of MRSA isolates were varied in different wards which partly reflected the fact that some patients, e.g., critically ill patients in ICUs, had a greater chance of becoming colonized or infected (28).
In conclusion, the accurate diagnosis of MRSA strains in hospitals, patients and health care workers is an important need. Also the dissemination of MRSA and MSSA strains with high resistance to different antibiotics in Tehran hospitals is a warning for public health. Accurate and continuous surveillance of antibiotic resistance patterns among S. aureus strains should be considered in health programs.