1. Background
Helicobacter pylori (H. pylori), a class I carcinogen, is a Gram-negative, flagellate bacillus, microaerophilic, spiral bacterium that colonizes the stomachs of approximately half the world’s population (1-4). H. pylori is one of the most common infectious diseases in the world. It colonizes about 50-60% of the world’s population. H. pylori infection is an important cause of chronic gastritis; it promotes peptic ulceration, and is a risk factor for gastric adenocarcinoma and maltoma. Although H. pylori infection always results in histological gastritis, only minorities of infected subjects develop an associated clinical disease. Major routes of transmission are either oral to oral or fecal to oral pathways. Iran is one of the countries where H. pylori infection is the most prevalent. The estimated prevalence of H. pylori infection is approximately 65% and thus it is a significant health problem. Eradication of H. pylori infection has become a wide clinical practice for H. pylori related diseases, but the results obtained appear to be controversial (5-10).
A large body of evidence emphasizes on the crucial role of antimicrobials in eradication of H.pylori and thereby reduction of the severity of gastric disease symptoms or complete recovery of patients. Most consensus guidelines are now recommending crucial antibiotics for the eradication of H. pylori in infected patients. The most commonly used treatments include bismuth salts, H2 receptor antagonists and proton pump inhibitors such as omeprazole, lansoprazole and pantoprazole. Amoxycillin, tetracycline, metronidazole and clarithromycin are the most effective therapies for this infection (11-13).
Meanwhile, the treatment of H. pylori infection does not always eradicate the organism. Antibiotic resistance is increasingly recognized as the contributing factor in 10-15% of patients in whom H. pylori eradication therapy fails. The prevalence rate of antimicrobial resistance to H. pylori varies with geographical regions (14-17). Determination of antimicrobial susceptibility is therefore important, particularly when treatment has failed. The present study aimed to improve our understanding of the Iranian regional variations in H. pylori antibiotic resistance rates in relation to gender, and to find the best antibiotic therapy for the eradication of H. pylori infections in Isfahan, Iran.
2. Objectives
The aim of the present study was to investigate the antimicrobial resistance of H. pylori to metronidazole, clarithromycin and amoxicillin.
3. Materials and Methods
3.1. Patients and Specimens
A total of 110 biopsy specimens were collected from patients with clinical symptoms of gastrointestinal disorders who were referred to the endoscopy unit at Al-Zahra Hospital, Isfahan, Iran between March to June 2011. The patients were not exposed to any prior antibiotics for H. pylori infection for the previous two weeks. Two pieces of antral gastric tissue were obtained from each patient; the first was used for rapid urease test and the second, was placed in sterile tubes containing brain heart infusion broth (Merck, Germany) supplemented with 20% glucose (Merck, Germany) and was immediately sent to the microbiology laboratory of the medical department for other processes. Subjects of this study were 66 women whose age were 21-58 years old (average age: 39) and 44 men whose age were 15-43 years old (average age: 29).
3.2. Isolation and Identification of H. pylori
The biopsy specimens were placed on the Brucella agar supplemented with 5% defibrinated sheep blood, 2% L-cystein (Merck, Germany), 10% fetal bovine serum (Sigma, USA), 10mg/L of vancomycin, 5mg/l of trimethoprim, 0.25 mg/L of polymyxin B and 5 mg/L of amphotericin B. The plates were incubated in a microaerophilic atmosphere (10% CO2, 6% O2, and 84% N2) by use of the MART system (Anoxomat, Lichtenvoorde, Netherlands) and relative humidity at 37°C for 3 to 5 days. Bacterial growth was identified by H. pylori colony morphology, Gram staining and positive biochemical reaction to catalase, oxidase and urease tests. If, the three tests were positive simultaneously, the sample was considered H. pylori positive. The isolates were sub-cultured on the brucella blood agar for 48 - 72 hours and stored at -80°C in aliquots of brucella broth supplemented with 20% (v/v) glycerol, 7% (v/v) fetal bovine serum and 2% L-Cystein.
3.3. Antibiotic Susceptibility Test
The susceptibility of the H. pylori isolates to metronidazole, clarithromycin and amoxicillin was examined by the epsilometer test (E-test, Biomerieux, France). A suspension equal to the McFarland tube no 3, was prepared for each isolate. The E-test strips for the antibiotics were aseptically placed onto the dried surface of inoculated agar plates (Mueller Hinton agar, Merck, Germany) and sheep blood (5% v/v), according to the CLSI instruction. The plates were incubated under microaerophilic condition at 35°C for 72 hours or longer until a visible inhibition ellipse was seen.
4. Results
Results were recorded as resistance according to the following interpretive criteria: for clarithromycin, a zone ≥ 1 µg/mL; for metronidazole a zone ≥ 8 µg/mL; for amoxicillin, a zone ≥ 0.5 µg/mL. These breakpoints were used based on the recommendations of the National Committee for Clinical Laboratory Standards (NCCLS) (11, 18-22).
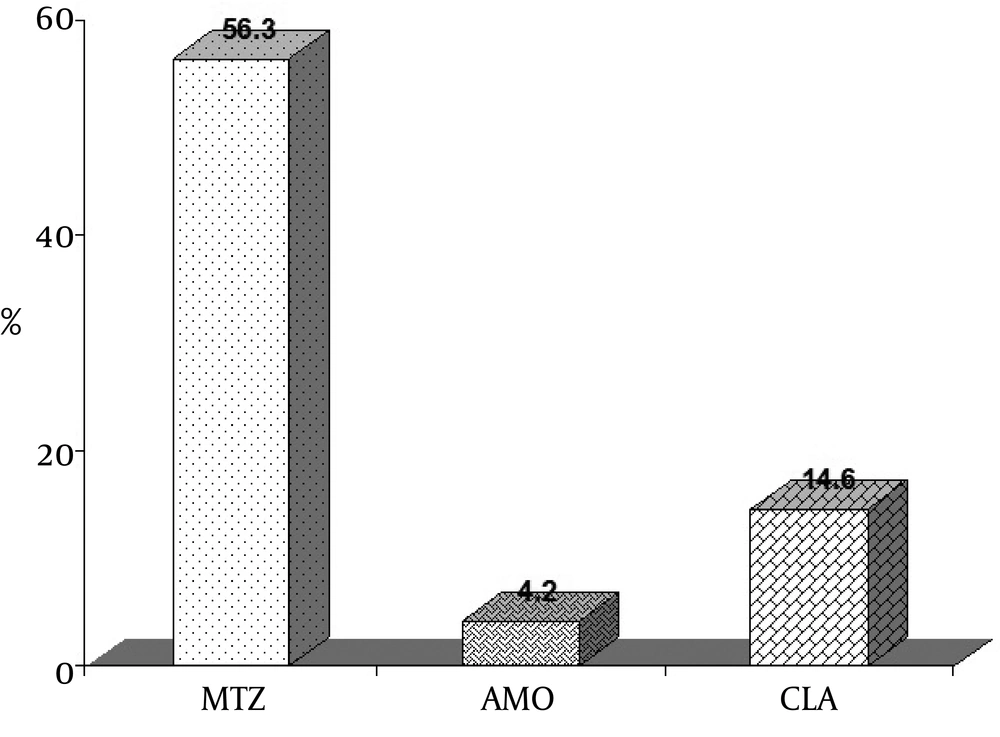
For patients in whom endoscopic biopsy specimens were taken for culture and antibiotic susceptibility testing, H. pylori grew in 48 of 110 (43.63%) cultures. Frequencies of resistance rates of 48 isolates to selected antibiotics were 56.3%, 14.6% and 4.2%, for metronidazole, clarithromycin and amoxicillin, respectively (Figure 1).
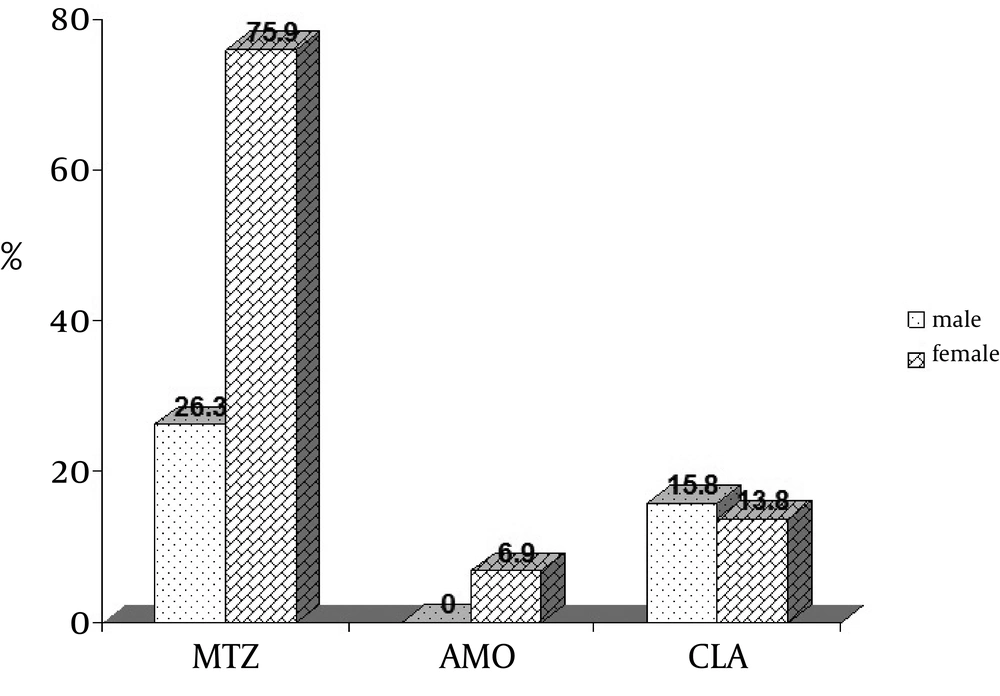
Frequency of resistance rates to selected antibiotics based on gender separation from which the strains were obtained is showed in Figure 2.
4.1. Statistical Analysis
The results of this study were analyzed by using χ2 test for comparing the means. Statistical analysis revealed that resistance to metronidazole was significantly higher in female than in male patients (P = 0.001). Concerning clarithromycin and amoxicillin, the percentage of resistance in female patients was 13.8% and 6.9%, respectively and in male patients was 15.8% and 0%, respectively, whereas, the differences in resistance to these two antibiotics among the isolated strains from both female and male were not significant, P = 0.58 and 0.36, respectively.
5. Discussion
H. pylori infect the majority of the adult population in developing countries including Iran. The rate of infection in Iranian adults according to serological data reaches 80% (23). Resistance to antimicrobial agents of H. pylori is of particular concern because it is a major determinant in the failure of eradication regimens. Antimicrobial drug resistance has been reported for nitro-imidazoles, macrolides, fluoroquinolones, amoxicillin, rifabutin and tetracyclines (24-27). Therefore, susceptibility testing of H. pylori is becoming increasingly important in order to curepatients properly.
A high rate of resistance to nitroimidazole (72-79%) was found throughout the study by Falsafi et al., these results are similar to frequencies reported for other developing countries (28). Antibiogram analysis by Mohamadi et al. of selected strains demonstrated 57% metronidazole resistance (29). In the study carried out by Siavoshi et al. 37.5% of H. pylori isolates were resistant to metronidazole (15). In a study performed by Khashei et al. in Isfahan, overall rate of resistance to metronidazole was 30% (23), while in our study which was performed four years later, this increased to 56.3% and indicates the importance of drug administration in order to prevent antimicrobial resistance.
Haghi Tomatari et al., evaluated the rate of resistance to metronidazole and reported a resistance of 64% for H. pylori isolates (14). Also, the rate of resistance to metronidazole in Shiraz decreased from 72.6% in 2007 to 44% in 2010 (22, 26). Data from Germany and France showed metronidazole resistance was about 40-50% and this data was contradicted data from China and Taiwan reporting 50-60% of resistance (29). In contrast, no resistance to metronidazole has been reported from Canada (27, 30). It is believed that the rate of resistance to metronidazole is increasing in our country and we should be aware of its disadvantage. Of course, this rate of resistance to metronidazole or other antimicrobial agents fluctuates in the world and it depends on poor socio-economics, life condition, geographical area, drug doses, period of treatment and excessive administration of antibiotics due to other infections.
Metronidazole resistance was more frequent in H. pylori isolates from female than male patients (75.9% versus 26.3%) and the result of our study is in agreement with data presented by Mansour et al. in Tunisia (67.8% in females against 32.2% in males). The same data was explained by Koletzko et al. in their study where they found that metronidazole was widely used in Africa and Asia to treat parasitic diseases and gynecological infections (31) and because of this reason; gender has been suggested to be a risk factor for resistance to metronidazole (32). Among macrolid antibiotics examined for eradicating H. pylori, clarithromycin plays a cardinal role (28).
The rate of resistance to clarithromycin has also fluctuated in different parts of Iran. For instance, in several studies performed in Tehran, capital of Iran, resistance rate to clarithromycin was as follows: in two studies, Fallahi et al., and Falsafi et al., reported the rate of resistance to clarithromycin as 4.16% and 21%, respectively (3, 28). In concomitant studies done in 2005 in various hospitals from Tehran by Siavashi et al. and Mohammadi et al. prevalence of clarithromycin resistance was 14.5% and 16.7%, respectively (15, 29) and that reflects the importance of diverse geographical areas.
In Tomatari et al. and Aslani et al. study, 23% and 14% of the isolates exhibited resistance to H. pylori, respectively (22, 33). In general, we can conclude that resistance rate of clarithromycin in Tehran has had an erratically upward trend.
In two other study in 2007 and 2010, carried out in Shiraz, south of Iran, by Kohanteb et al. and Farshad et al. prevalence of resistance rate was 9.4% and 5%, respectively (22, 26). Reports by Kargar et al. indicated that the frequency of clarithromycin resistance was 22.62% in Shahrekord and this result is relatively in agreement with reports by Tomatari et al. in Tehran (34). In Isfahan , Khashei et al. conducted a study that reported 6.25% of resistance (23). Since the Khashei et al. study, this amount has dramatically surged to 14.6%, as observed by our study. So, it can be concluded that the rate of resistance to clarithromycin in Isfahan has increased during the past 4 years, but not as much as Tehran. Rates of resistance to clarithromycin substantially vary in different parts of the world. It has been reported that immense alterations in the resistance rates exist between the northern and southern parts of Europe. In addition, frequency of resistance of H. pylori strains to clarithromycin was 5 to 6% in Korea (35), 4.5% in Hong Kong (36), 11% in London (37), 2.14% in Malaysia (38), 14.6% in Tunisia (31) and 8% in Brazil (39).
Several studies were done in Japan by their researchers during 2002 and 2011,reporting that the frequency of clarithromycin resistant strains was 11-12% (2002), 83.9% (2004) and 36.1% (2010) and ultimately it reached 38.8% in 2011 (16, 34, 40, 41). In other studies in Poland, rate of resistance to clarithromycin, evaluated during 1998, 2005 and 2008 had an increasing trend form 9.1% to28% and 18.4%, toward to 1998, respectively (39, 42, 43). Although clarithromycin is an effective antibiotic and it is mostly prescribed to combat H. pylori, in agreement with consensus conferences (2011), this antibiotic is not advised when the proportion of resistance obtained is between 15% to 20% (41). In contrast to metronidazole, there was no significant difference between resistances to clarithromycin in male and female (15.8% versus 13.8%). As compared to metronidazole, resistance to clarithromycin did not increase so much, but this amount vary is of concern. Resistance to clarithromycin have not increased so much as compared to metronidazole, but this amount vary is of concern.
Amoxicillin is the only β-lactam used to treat H. pylori infection and it is included in most current therapeutic regimens (26). Resistance to amoxicillin (2003) was observed in 8.33% of the isolates, reflecting the importance of its administration in our country (3). Subsequent to this report, several investigations were done in Tehran, which considered amoxicillin resistance. Rate of amoxicillin resistance of H. pylori strains in 2004 was reported as 27% in children while two simultaneous studies in 2005 revealed resistance rates of 7% and 1.6%. Other studies showed resistance rates of 2.5% and 2.4% for 2007 and 2008, respectively (14, 15, 28, 29, 33). Comparison of these studies revealed that the rate of resistance to amoxicillin is more in children than adults. In two studies performed in Shiraz during 2007 and 2010, rate of resistance to amoxicillin had a slight decline from 20.8% to 20% (22, 26). Even though, results of a survey (2008) in Isfahan illustrated a rate of 2.5% (23), the overall rate of resistance to amoxicillin is approximately two-folds greater and has reached 4.2% in our study and no significant difference was seen between resistance to antibiotics and gender. Although there was no observed resistance to amoxicillin in Japan (16), Poland (4, 39), Tunisia (31) and Brazil (42), high resistance rates have been reported for South Korea (18.5%) and Indonesia (19.4%) in 2004 and 2006 (33).
Finally, we can conclude that the rate of resistance to amoxicillin is increasing and this rise is a serious alarm as this drug is the most effective antibiotic for eradicating the organism. The majority of studies were done in Iran, determining methods for antibiotic resistance were disk diffusion or agar dilution, but we used E-test strips that are more accurate than these methods. Albeit, E-test is an expensive technique in Iran and is the best technique for a wide spectrum of antimicrobial agents for determining reliable MIC that are recommended to be use for in vitro susceptibility tests, routinely. Due to the importance of amoxicillin in treatment of H. pylori infection and eradicating of this pathogen, increased or decreased MIC from 0.5 µg/mL in Iran must be under uninterrupted surveillance.
Knowledge of the antibiotic susceptibility patterns in our setting allows us to be more cautious in the choice of first-line agents. Information on antibiotic susceptibility profile plays an important role in empirical antibiotic treatment and management of refractive cases. Susceptibility testing of H. pylori isolates in different geographical areas is advisable because it is an aid for selection of optimal therapy regimens. Actually, it is vital to be aware of local resistances in every country in order to prescribe effective antibiotics for eradicating this organism.
In general, combination of a quadruple regimen for metronidazole is still recommended for eradication of this pathogen in patients with gastroduodenal ulcers(43, 44) and has been prescribed against H.pylori in Iran and other countries (45), but using metronidazole in our region, Isfahan can lead to eradication failure in clinical therapies due to having the highest rate of resistance. The antimicrobial agents prescribed in Isfahan against H. pylori by our gastroenterologists for first lines of treatment are amoxicillin, azithromycin, omeprazole and bismuth citrate and for second line of treatment are clarithromycin, tetracycline and furazolidone. Amoxicillin and clarithromycin are prescribed for eradicating H. pylori in first and second line of treatment because of having a lower rate of resistance than metronidazole, respectively. In conclusion, our data suggest that the E-test appears to represent an excellent alternative and reproducible method for determining the antimicrobial susceptibilities of H. pylori strains. Also, it is recommended that continuous surveillance of antibiotic resistance to H. pylori and determination of MIC should be assayed annually in order to prescribe and achieve better treatment for eradicating the organism.