1. Background
Leishmaniasis is among zoonotic diseases with the highest importance worldwide. It is a sand fly-transmitted protozoan parasite whose promastigotes are transmitted by the bite of infected female phlebotomine. Leishmania is an obligate intracellular protozoon in the order of kintoplastida that enters the macrophages of liver, spleen and bone marrow of vertebrate hosts. Leishmania has more than 30 different species (1) and three forms of clinical diseases, which depend on the species of the parasite: cutaneous leishmaniasis, mucocutaneous leishmaniasis, and visceral leishmaniasis (2).
At least three species of Leishmaniadonovani complex can cause visceral leishmaniasis. These species have clinical, biochemical and epidemiological differences. L. infantum is among such species.Visceral leishmaniasis in Iran mostly involves children below 2 years of age (3) and are responsible for a wide spectrum of clinical manifestations in humans, particularly in children up to 12 years of age and immunocompromised patients(4). Most people infected with L. infantum have the long lasting symptoms of fever, cough, weight loss, enlarged spleen and enlarged liver. Furthermore, death rate in patients who get no treatment is more than 90% (5).
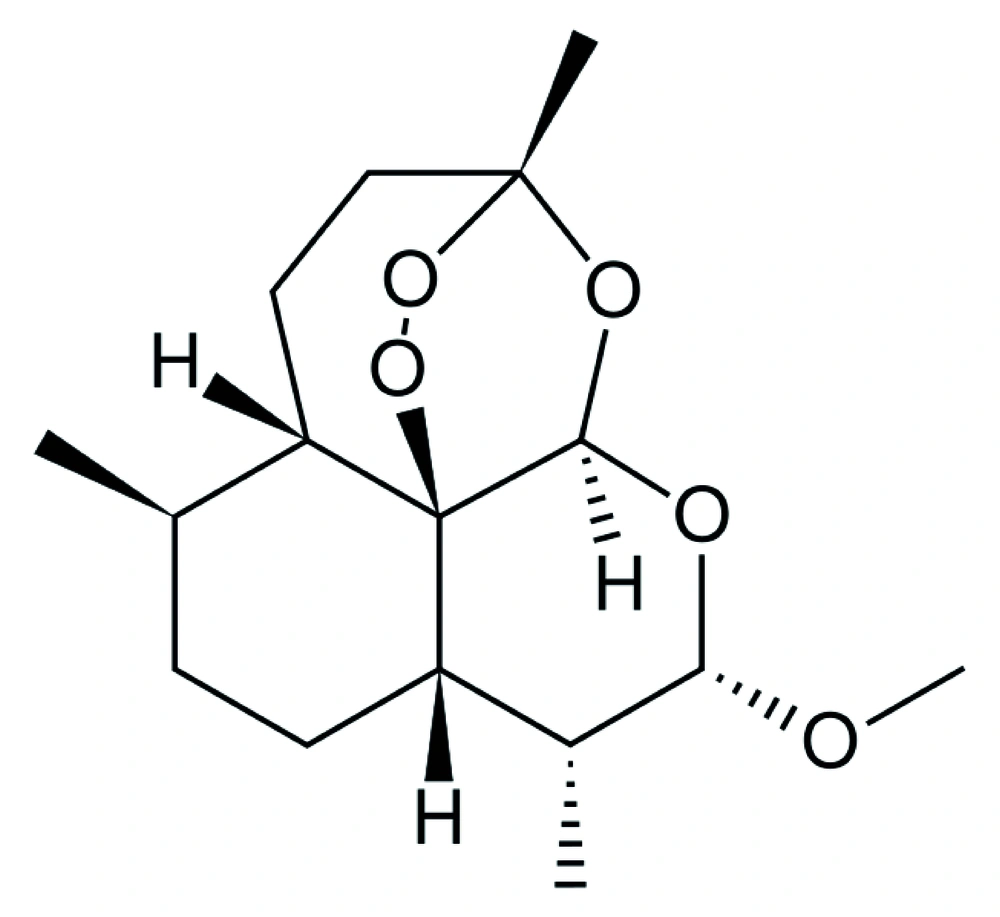
There are many chemical drugs such as sodium stibogluconte (pentostam), meglumine antimonite (glucantime), paramomycin, amphotericin B, desoxycolate and lipid formulations of amphotericin B, which are chosen for effective treatment of visceral leishmaniasis ( 6 ). Miltefosine is an alkyl phosphoril choline employed in 1987 against L. donovani ( 7 ). This chemical was tested in 1998 on L. infantum as well ( 8 ). Concerning some drugs, following any strategy has some limitations because of the drugs’ unacceptable toxic effects and emerging threat of drug resistance in some countries (9). Over the past 25 years, artemisinin, an herbaceous drug, and its derivatives have been used for treatment and growth inhibition of some parasites (10). Simultaneously, antitumoral effects of this drug have been evaluated. One of the derivatives of artemisinin is artemether (Figure 1) which so far has been exploited for treatment of parasitological diseases such as malaria, Schistosoma japonicum, S. haematobium , S. mansoni , Fasciola hepatica and Fasciola gigantic (11,12). Trematodes need hemoglobin of the host as artemether can be activated by heme which makes it capable of producing free radicals with concomitant adverse effects on the parasite (13). The anti-parasitic activity of artemisinin and its derivatives is related to the endoperoxide bridge in their structures (14). Another study has also demonstrated that artemisinin is effective on visceral leishmaniasis (15).
2. Objectives
The current study mainly aimed to evaluate the Antileishmanial Effect of Artemether on L. infantum In Vitro and In Vivo and also to evaluate the cytotoxic effect on this parasite via apoptosis-related mechanism.
3. Materials and Methods
3.1. Artemether Preparation
Artemether was purchased from Exim Pharm Co. (US). Stock solutions of the drug were freshly prepared at concentration of 1 mg/mL (16).
3.2. In Vitro Conditions
3.2.1. Parasite Culture
L. infantum (MHOM/TN/80/IPI1) was obtained from Pasteur Institute of Iran. Promastigotes were cultured in RPMI 1640 (Gibco, UK) enriched with FCS 20% (fetal calf serum) (Gibco, UK), 100 IU/mL of penicillin, and 100 μg/mL of streptomycin to have adequate promastigotes.
3.2.2. Promastigote Assay
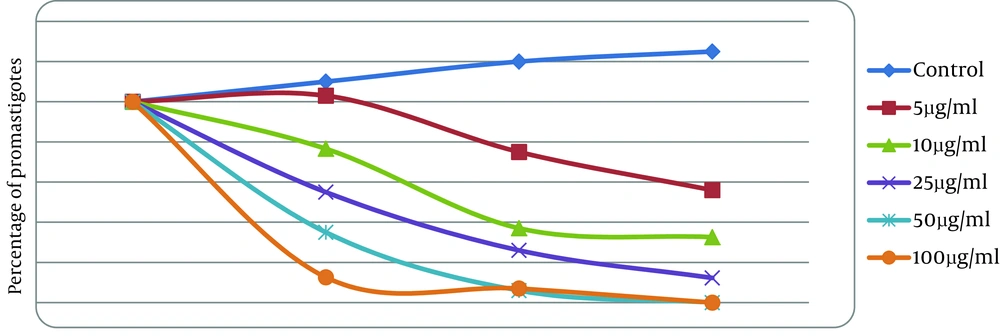
To evaluate 50% inhibitory concentration (IC50) of artemether on L. infantum, microtitration plate was used. Total volume of each well was 200 μl, containing parasite (106 promastigotes/mL) culture medium (17) and antibiotic. Also, the wells were treated with artemether in concentration range of 0, 5, 10, 25, 50, and 100 μg/ml (each one was in triplicate). The plate was incubated at 24◦C and evaluated each 24, 48, and 72 hours with microscope. The evaluation of IC50 was done according to the results of 24 h. IC50 was measured by linear regression of plots.
3.2.3. Flowcytometry Analysis
3.2.3.1. Apoptosis Assessment With Flowcytometry
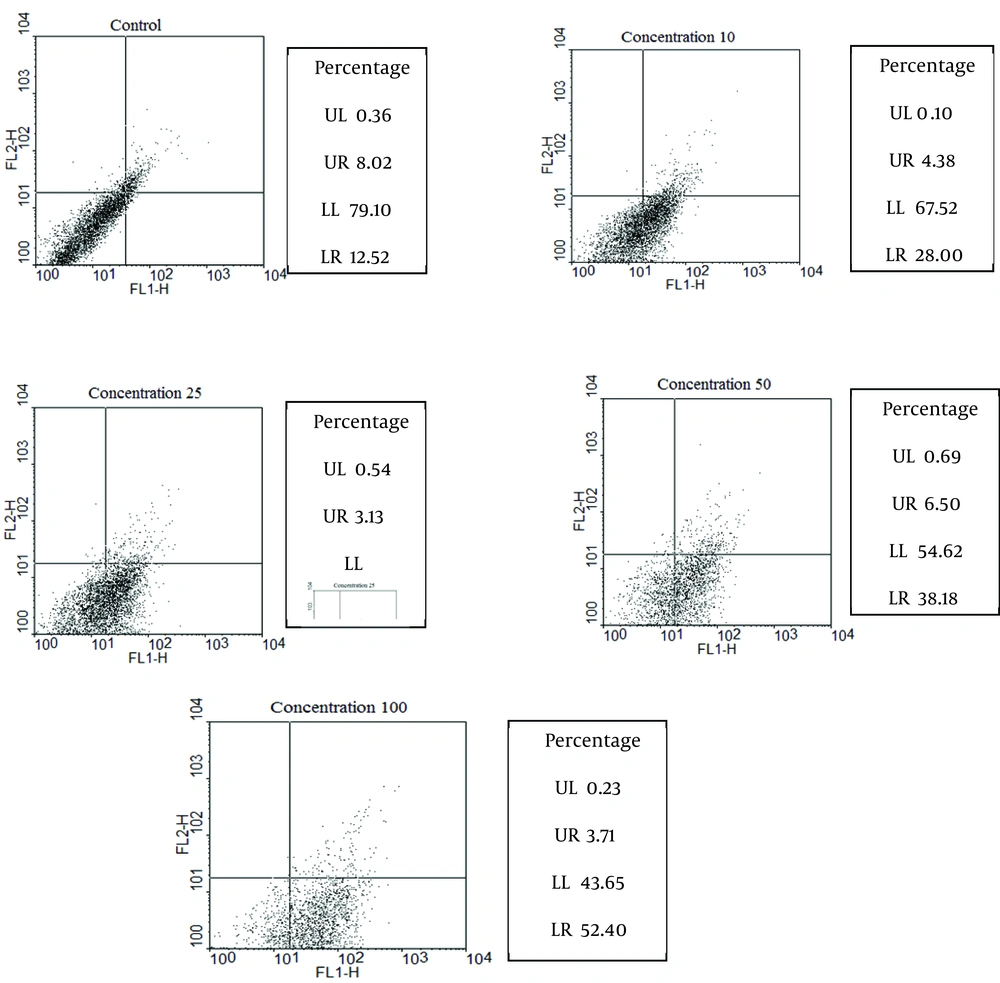
Promastigotes (106/mL) in logarithmic phase were cultured in RPMI 1640 enriched with FCS 20%, 100 IU/ml of penicillin, and 100 μg/mL of streptomycin. Total volume of each well was 1000 μl, containing parasite, culture medium, and antibiotic. Moreover, the wells were treated with artemether in concentration range of 0, 10, 25, 50, and 100 μg/mL. The plate was incubated at 24◦C and evaluated after 72 h with flowcytometry. Annexin-FITC, V FLUOS Staining Kit (Roche, Germany) was used to detect apoptotic and necrotic cells according to the manufacturer's protocol. Promastigotes were washed in cold phosphate-buffered saline (PBS) (×2) and centrifuged for 10 min at 1400 g and promastigotes counted (1 ×106/mL). Afterwards they were incubated for 15 min at room temperature in darkness, with 10 μl of annexin-FITC, V FLUOS in the presence of 10 μl PI (propidium Iodide). The samples were then analyzed with FACS Calibur flowcytometer (Becton, Dickinson and Cell Quest software). Finally, the percentage of positive cells was determined for each sample.
3.3. In Vivo Conditions
3.3.1. Infecting the Mice With Parasite
Experiments were conducted with 45 female, 8-10 weeks oldBALB/c mice, which were obtained from Razi Institute of Iran. On day 0, each mouse was inoculated parenterally with 107 promastigotes. The mice were divided randomly into two groups, i.e. control and experimental.
3.3.2. Parenteral Treatment with Artemether
In the experimental group, 24 mice were infected parenterally with 107 promastigotes. The treatment started 18 days after inoculation of promastigotes with 0.625 mg/kg of parenterally-treated artemether (these doses were calculated according to IC50 results, according to the mean weight for each mice were 25 g, then drug concentration was calculated 0.625 mg/kg ). These mice were subsequently divided into four groups and each group was treated for 4, 8, 12 and 16 days. Twelve mice were selected as the control group and divided into four groups to compare them with experimental groups.
The parasite burden calculated according modified method of Ahmed et al (18). The mice in each group of parenteral treatment and control group were sacrificed after each period of treatment and whole spleen (about 0.2 gr) as well as a piece of the left lobe of liver (about 0.28 gr) were excised and homogenized in RPMI 1640 (Gibco, UK) enriched with FCS 20% (fetal calf serum) (Gibco, UK), and 100 IU/mL of penicillin. About 20 μl of each sample was counted with Neubauer chamber and light microscope to count the parasite rate.
3.3.3. Oral Treatment With Artemether
Six mice were chosen and treated with artemether during 3 days, and the drug was administered every 6 h orally (0.625 mg/kg). Oral feeding was done by gavage in 200 µl volume. The amount of artemether for oral treatment was calculated according to suitable level of artemether in the serum due to the half-life time and IC50 results. Three mice were chosen as control group too. The mice in oral treatment and control group were sacrificed after the period of treatment and the parasite rate was evaluated according to parenteral treatment groups.
3.3.4. Data Analysis
Data were summarized by mean and standard deviation (SD) and by Mann-Whitney test; P values of < 0.05 and < 0.01 were considered significant.
4. Results
4.1. In- vitro Assay
4.1.1. Cytotoxic Effect of Artemether on L. infantum Promastigotes (MHOM/TN/80/IPI1)
The results showed that growth assay of L. infantum promastigotes in the presence of different concentrations of artemetheris was time and dose dependent.IC50 was measured as 25 μg/mL after 24 h (Figure 2).
4.1.2. Flowcytometric Analysis After labeling With Annexin-V FLUOS
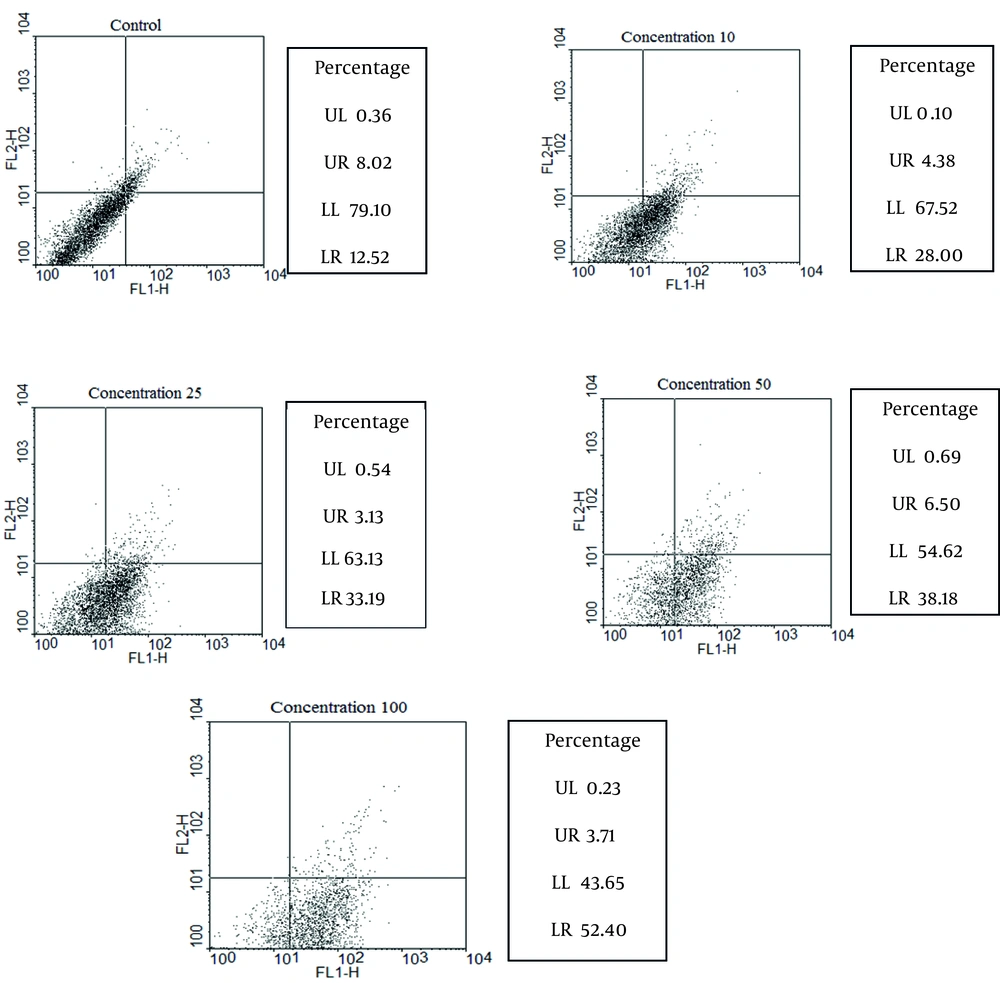
The percentages of apoptotic, necrotic, and surviving cells were determined at each concentration of artemether after 72 h and are coming in (Figure 3).
The control group was not treated with artemether, while promastigotes were treated with 10, 25, 50 and 100 μg/mL of artemether, respectively. Lower right region (LR) belongs to apoptotic cells (annexin-positive) and upper left region (UL) belongs to necrotic cells (PI-positive). Upper right region (UR) belongs to banded cell region with annexin and PI, and lower left region (LL) belongs to survived cells. FL2 is propidium iodide and FL1 is Anaxine V.
4.2. In-vivo Assay
4.2.1. Parenteral Treatment With Artemether
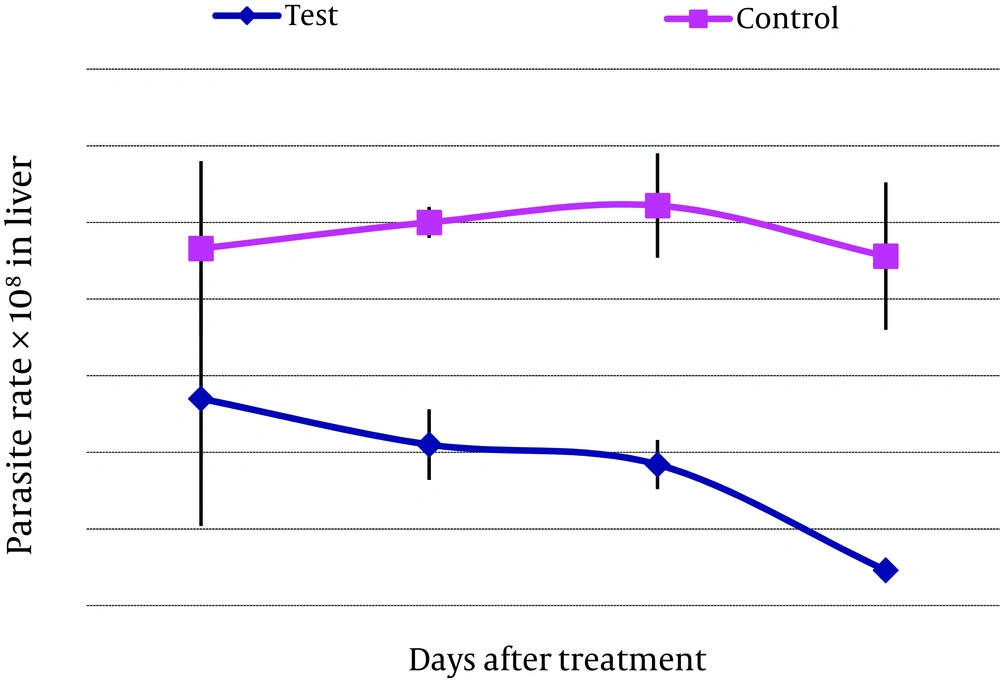
This study was designed to evaluate the efficacy of artemether for the parenteral treatment of an experimental visceral leishmaniasis caused by L . infantum (Figures 4 and 5) .
For parenteral treatment of mice with artemether, 24 mice were treated during 4-16 days. Parenteral artemether treatment (0.625 mg/kg) on mice inoculated with 107 promastigotes and subsequent evaluation of parasite rate in liver and spleen of the subjects revealed no significant decrease in parasite rate in the group treated for 4 days in comparison with the control group. Meanwhile, on days 8, 12 (P < 0.05) and 16, reduction of parasite rate in liver and spleen was significant (P < 0.01) (Table 1). For oral treatment of mice with artemether, six mice were chosen and treated with artemether during three days. Every 6 h, the drug was administered orally (0.625 mg/kg). Three mice were included in the control group. Oral artemether treatment of the mice inoculated with 107 promastigotes showed a remarkable decline in parasite rate in liver and spleen of mice (in liver pv .018 and in spleen pv.034) (Table 1).
| Tissue | Days After Treatment by Artemether 0.625 mg/kg (Parenteral Injection) | Oral Treatment by 0.625 mg/kg of Artemether for 3 Days | |||
|---|---|---|---|---|---|
| 4 Days, Mean ± SD | 8 Days, Mean ± SD | 12 Days, Mean ± SD | 16 Days, Mean ± SD | ||
| Liver | |||||
| Test | 1.35 ± 0.83 | 1.05 ± 0.23 a | 0.92 ± 0.16 a | 0.23 ± 0.03 b | 0.18 ± 0.06 b |
| Control | 2.33 ± 0.57 | 2.50 ± 0.10 | 2.61 ± 0.34 | 2.28 ± 0.48 | 2.04 ± 0.24 |
| Spleen | |||||
| Test | 1.92 ± 0.92 | 1.80 ± 0.2 a | 1.26 ± 0.06 b | 0.76 ± 0.02 b | 0.33 ± 0.05 b |
| Control | 4.10 ± 0.70 | 4.06 ± 0.30 | 3.52 ± 0.32 | 3.80 ± 0.26 | 3.69 ± 0.23 |
aSignificant differences with control group were designated as (P < 0.05).
bSignificant differences with control group were designated as (P < 0.01).
5. Discussion
As there isn’t any effective vaccine against visceral leishmaniasis, finding new safe methods for treatment is very important. In this regards, the plant extractions with high effect on Leishmania parasites, and low cytotoxicity for human cells are favorable. In the current study, treating visceral leishmaniasis was directed toward the eradication of parasites and reduction of the parasite rate in the spleen and liver of BALB/c mice. Artemether is among the derivatives of artemisinin which is extracted from the plant Artemisia annua and is a new anti-malarial drug. It is used for treatment against the erythrocytic stages of chloroquine-resistant Plasmodium falciparum and for cerebral malaria. Probably anti-malarial specificity of artemether can be attributed to reductive activation of peroxide bond by parasite hemoglobin digestion which gives rise to carbon-centered free radicals (14). Artemether might act against Fasciola spp. similar to anti-plasmodial mechanisms of action which had been described to include an interference with the parasites’ sarcoplasmic/endoplasmic reticulum Ca2+-ATPase PfATP6 (SERCA), disruption of the parasite’s mitochondrial function, modulation of host immune function, and inhibition of angiogenesis (14).
The current study demonstrated the best and the most effective dose of artemether on L. infantum (MHOM/TN/80/IPI1). It was measured by the promastigote assay in order to determine 50% inhibitory concentration (IC50) of the drug. IC50 was measured as 25 μg/mL after 24 h. Apoptosis as a form of programmed cell death involves a biochemical cascade which includes proteins such as Bcl-2, Bax, apoptotic protease activating factor-1 (Apaf-1), caspases such as caspase-9, caspase-3, and caspase-7, as well as proteins involved in the digestion of proteins, degradation of DNA, and phagocytosis. Fluorescein-conjugated annexin-V was used to detect externalized phosphatidylserine as it has a high binding affinity to this phospholipid component. Annexin-V FLUOS shows the percentage of apoptotic, necrotic, and surviving cells. Khademvatan et al., had used Annexin V- FLUOS staining to study apoptotic properties of the Allium sativum extract using FACS flow cytometry.
The results revealed that A. sativum extract as a plant product induced apoptotic phenomenon in promastigotes of L. major. In their study the percentage of apoptosis , using 37 µg/mL as IC50 was 86.11%, whereas in the current study the percentage ofapoptosis using 25 µg/mL as IC50 was 33.19 (19). Soflaei et al., used antimony sulfide NPs, IC50 and promastigotes was calculated 50 μg/mL. In that study the percentage ofapoptosis using 50µg/mL as IC50 was 39.8% (20). Half-life of artemether in the serum is 3 h and the current study used artemether every 6 h orally. Results of the current study indicated that 0.625 mg/kg/6h was the suitable level of artemether in the serum. Oral artemether treatment of mice during 3 days and every 6 h (0.625 mg/kg) was more significant than that ofday 16. Both oral and parenteral administrations of artemether are effective. In addition, oral treatment is an effective and simple method and may be used as a new method for treating visceral leishmaniasis.