1. Background
Urinary tract infection (UTI) represents as one of the most common diseases encountered in medical practices these days and encompasses a broad range of clinical fields that are associated with a common finding of positive urine cultures (1, 2). It is the second most common infectious presentation in community. There are an estimated 150 million UTIs per year, worldwide (3, 4). The clinical significance of the infections is due to its high mortality rate, malignant and chronic hypertensions and the chronic renal failures followed by chronic pyelonephritis (5, 6). The severity of UTI depends on the bacteria virulence and susceptibility of the host (7). This infection varies in patients with different gender, age, and presence of associated genitourinary abnormalities (8).
UTI is the most common infectious diseases among women (2, 9, 10). Nearly 1 woman out of 3, will have at least 1 episode of UTI requiring antimicrobial therapy by the age of 24 years, and almost 50% of all women will experience at least 1 episode of UTI during their lifetime (11). UTIs are also the most common infections in hospital and extended-care settings, it has been reported that 2% – 3% of admitted patients are suffering from UTI (1, 2, 12).
The etiology of UTIs and the antibiotic susceptibility of urinary pathogens, both in community and hospitals, have been changing over the past years and recently, the antibiotic resistance has become a major global problem (1, 3, 7, 9). There are many reasons for this problem, including antibiotic consumption while feeding the t animal, inappropriate prescription of antibiotics and poor infection control strategies (1). Due to constant varying of isolated UTI causative microorganisms and antibiotic sensitivity pattern, we recommend to determine bacterial sensitivity in populations every year.
2. Objectives
This study is aimed to detect and also compare the frequency and drug resistance pattern of Gram negative bacteria isolated from patients with community acquired UTIs, in Isfahan (Iran).
3. Patients and Methods
3.1. Sample Collection and Analysis
In this cross-sectional descriptive study, we investigated the urine culture and antibiotic sensitivity of the isolated organisms of the 702 urine samples (476 females and 226 males) of the people refers to medical centers in Isfahan city from June to September 2011. Sampling was performed simply and accidentally. The patients were referred to laboratory or hospital by physicians for detection of urine infection and or abnormality in urine. The study parameters were included the age, sex, bacterial species and bacterial sensitivity or resistance to antibiotics. Isolates were collected without age and sex limitations. Sampling was done from the midstream specimens of urine.
Semi quantitative urine culture using a calibrated loop was performed. Samples were inoculated on a blood agar (Merck, Germany), Mac Conkey agar (Merck, Germany) and Eosin Methylen Blue agar (Merck, Germany) plates. Positive culture was defined as if the bacterial colony count was equal or more than 105 CFU.mL-1 (4, 11). The species of all isolates were identified t by standard biochemical methods (3). The sampling was blinded and it was not known whether the submitted urinary isolates came from patients with symptomatic upper or lower UTIs or from patients with asymptomatic bacteriuria; however; all submitted isolates had be considered significant by the participating laboratory, identified to species level.
3.2. Antibiotic Susceptibility Test
Antimicrobial susceptibility test was performed using disc diffusion method as described by Clinical and Laboratory Standards Institute (CLSI) (13). Briefly, colonies taken from overnight growth on 5% sheep blood agar (16 – 20h. at 35°C) were re-suspended in Mueller-Hinton broth (Merck, Germany).The turbidity of the suspension is adjusted to an equivalent 0.5 McFarland .This suspension was used to inoculate on Mueller-Hinton agar (Merck, Germany) plates. Cephalexin (CN), Cipropheloxacin (CP), Nalidixic acid (NA), Nitrofurantoin (FM), Trimet-sulpha (SXT), Cefaxim (CFM) and Cotrimoxazol (CTX) (Padtan Teb, Iran) were placed on Mueller Hinton agar .Gram-negative bacilli were incubated at 35 °C for 16–20 h. and then the inhibition zone was measured and compared with data of Inhibition Zones of Test Cultures by CLSI. Data were interpreted by the percent of susceptible, intermediate, or resistant isolates as defined by CLSI breakpoint interpretative Criteria (13).
3.3. Statistical Analysis
Data were analyzed by SPSS software version 16.0, and the Chi-square test, logistic regression and Independent samples t-test performed.
4. Results
Of the 702 urine samples processed (476 females and 226 males), 203 samples (28.92%) were positive for urine culture and had UTI. The mean age was 37.07 ± 22.2 years (range from 1 month to 93 years). The prevalence of UTI was observed in females 32.35% (154 persons) and males 21.68% (49 persons). This difference was significant statistically (P ≤ 0.01). The infection incidence in women was 1.74 times more than men (95%, CI: 1.2 -2.53, P≤ 0.01); so 1.8% of infection fluctuation was justified by gender (Table 1).
| Age, y | Male | Female | ||
|---|---|---|---|---|
| UTI Positive, No. (%) | UTI Negative, No. (%) | UTI Positive, No. (%) | UTI Negative, No. (%) | |
| 0 – 10 | 4 (13.3) | 26 (86.7) | 20 (31.7) | 43 (68.3) |
| 11 - 30 | 7 (14) | 43 (86) | 44 (25.7) | 126 (74.3) |
| 31 - 50 | 14 (20.6) | 54 (79.4) | 32 (24.8) | 97 (75) |
| 51 ≤ | 24 (30.8) | 54 (69.2) | 58 (51.3) | 55 (48.7) |
| Sum | 49 (21.68) | 177 (78.32) | 154 (32.35) | 322 (67.65) |
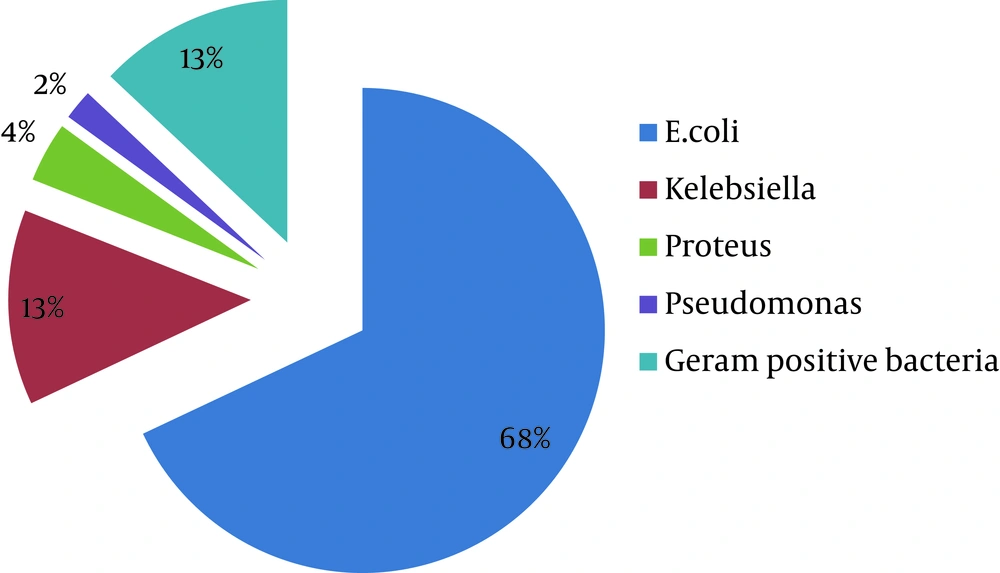
Patients were categorized into four different age groups: ≤ 10 years, 11–30 years, 31-50 years and ≥ 51 years (Table 1). Accordingly to Table 1, UTI is more frequent in older patients , so as much as the patients were elder, the rate of infections was higher. The prevalence of UTI was 28.92 % (95%, CI: 25. 52% -32.32%). The mean ages of patients with and without infection were 44.07 and 34.28, respectively. This difference was statistically significant (P ≤ 0.001). The mean age of patients with and without infection was 42.42 ± 2.11 and 32.39 ± 1.04 in women respectively. This difference is statistically significant (P ≤ 0.001). In men, it was 1.67 ± 37.73 and 49.27 ± 3.22, respectively. This difference was statistically significant (P ≤ 0.001). The most microbial agents leading to UTI were Escherichia coli representing 68% (n = 138) (Table 2), followed by Klebsiella 13% (n = 27), Proteus 4% (n = 8), Pseudomonas 2% (n = 4) and Gram positive 13% (n = 26) bacteria (Figure 1).
The antimicrobial strength of seven antibiotic agents against the most three frequent UTI pathogens was investigated (Table 3). Among the antibiotics, FM had the widest coverage against E. coli isolates (80 %) followed by CTX (65%), CP (57%), SXT (56%), CFM (44%), NA (32%) and CN (21%), respectively. The highest resistance rates against NA (63%) and SXT (58%) were observed among these isolates. For Klebsiella isolates, the antibiotic susceptibility prevalence is as follow: CTX (74%), CP (70%), CFM (63%), NA (52%) SXT (48%) CN(30%) and Fm (19%). These isolates had the highest resistance potency against SXT (48%) and CN (33%). Among Proteus isolates, CFM , CP and NA were the most active antimicrobial agents, with 89% , 89% and 78% effectiveness, respectively; also STX (22% ) and CTX (22%) shown the less antimicrobial activity against Proteus isolates.
| Age, y | E. coli (138) 68% | |
|---|---|---|
| Male, No. (%) | Female, No. (%) | |
| 0 – 10 | 3 (16.7) | 15 (83.3) |
| 11– 30 | 3 (11.2) | 24 (88.8) |
| 31– 50 | 10 (32.3) | 21 (67.7) |
| 51 ≤ | 18 (29.04) | 44 (70.96) |
| Antibiotic | Bacteria, No. (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Proteus, 9 (4) | Klebsiella, 27 (13) | E. coli, 138 (68) | |||||||
| R | I | S | R | I | S | R | I | S | |
| CTXa | 2 (22) | 1 (11) | 6 (67) | 5 (19) | 2 (7) | 20 (74) | 40 (29) | 8 (6) | 90 (65) |
| SXT | 2 (22) | 1 (11) | 6 (67) | 13 (48) | 1 (4) | 13 (48) | 80 (58) | 2 (1) | 41 (56) |
| NA | 2 (22) | 0 (0) | 7 (78) | 8 (30) | 5 (18) | 14 (52) | 86 (63) | 7 (5) | 44 (32) |
| CN | 1 (11) | 8 (89) | 0 (0) | 9 (33) | 10 (37) | 8 (30) | 47 (34) | 61 (45) | 29 (21) |
| CP | 1 (11) | 0 (0) | 8 (89) | 3 (11) | 5 (19) | 19 (70) | 54 (39) | 5 (4) | 79 (57) |
| FM | 2 (22) | 6 (67) | 1 (11) | 6 (22) | 16 (59) | 5 (19) | 8 (6) | 20 (14) | 110 (80) |
| CFM | 1 (11) | 0 (0) | 8 (89) | 8 (30) | 2 (7) | 17 (63) | 47 (34) | 30 (22) | 61 (44) |
In Table 4 and Figure 2 the rate and percent of resistance E. coli isolates to antibiotics was shown. According to these, more than half of isolates are resistance to 2 or more antibiotics used in this research.
| MDR of E. coli Isolates | No. |
|---|---|
| Total Sensitive | 33 |
| Resistance to 1 antibiotic | 13 |
| Resistance to 2 antibiotics | 31 |
| Resistance to 3 antibiotics | 15 |
| Resistance to 4 antibiotics | 8 |
| Resistance to 5 antibiotics | 14 |
| Resistance to 6 antibiotics | 20 |
| Total Resistance | 4 |
5. Discussion
It is important that clinicians are aware of the regional antibiotic resistance rates before initiating experiential antimicrobial therapy for UTI treatment, as it is well-described that urinary infection with a resistant pathogen is more likely to lead to bacteriological/clinical failures(6).
In this study the prevalence of antibiotic resistance of bacterial isolates was investigated. This study was done in Isfahan province, Iran. In this survey UTI in women showed a higher prevalence. In the other studies, like extensively reported results (1, 11), this higher prevalence of UTI is due to the anatomic and physical factors in women (11). The highest rate of infection was seen in age group more than 50 years old (29% male and 71% female).
In some studies, UTI was more detected in babies and adults ( 5 , 14 - 17 ) but in our study it was different. These results could be due to the increased level of hygiene and public culture and knowledge. Just as shown in Table 2, E. coli (68%) is the most frequent agent causing UTI. This bacterial agent was reported repeatedly as the most causative agent of UTI (1 , 3 , 4 , 11 , 18 ). The other Gram negative and Gram positive bacteria can also be leaded to UTI. Antibiotic resistance is a major clinical problem in treating infections caused by these microorganisms (3 ). The resistance to the antimicrobials has increased over the years. Resistance rates vary from country to country (3 ).
The impudence of age, gender and geographic location has previously been shown the amount of antibiotic resistance of urinary isolates (6 ). According to Table 3 the highest antibiotic resistance of E. coli isolates was related to NA (63%) and STX (58%). The most effective antibiotics against E. coli were FM (80%),CTX (65%) and CP (56%). The best response to antibiotic therapy was related to FM (80%). The rate of resistance to this antibiotic in our study was reported 6% and in North American and Canada was reported 1.1% and 1.9%, respectively (4 , 6 ). In a study in Tehran, Iran, the sensitivity to this antibiotic was reported 86% and introduced as a selected antibiotic for UTI treatment (5 ); however in another study in Hamedan, Iran, E. coli isolates were resistance (42.1%) to this antibiotic (19 ).
Despite extensive use of FM for many years, this antibiotic still possesses an excellent activity against urinary isolates of E. coli (6, 7, 11, 16, 17, 19); however, compared with fluoroquinolones, the FM is associated with lower treated rates and more side effects in the treatment of acute cystitis (11, 18); also, this antibiotic was poorly tolerated by children and old patients and cannot be used for pyelonephritis (5). In our study, after FM, the E. coli isolates were showing the least resistance to CTX. The rate of resistance to this antibiotic was in Europe (14.1%), USA (18.6%), Iran (29%), Spain (33%), Senegal (55%), Taiwan (56%) and India (75%) (3). In another study in Iran, E. coli isolates were resistance to this antibiotic (19). Resistance, particularly to traditional first line agents such as quinolones and SXT, has increased substantially across Iran. NA is an antibiotic from the first generation of queinolones but nowadays resistance to this antibiotic was reported repeatedly (7, 19).
Resistance was reported in Iran and also in our survey(19). CP is an antibiotic from the second generation of flouroqueinolones. According to this subject the functional mechanism of flouroqueinolones is better than FM, so the consumption of these antibiotics for UTI therapy is recommended. Resistance to this antibiotic is also increasing (6, 7, 11, 19). In studies in Tehran and Hamedan respectively 95% and 93.4% of bacterial isolates were sensitive to CP but in our study only 57% of E. coli isolates were sensitive to this antibiotic. In some studies CP was the selected antibiotic for treatment of all of UTI pathogens (11).
Klebsiella isolates were shown the least resistance antibiotic (11%) to CP but in the other regions of Iran such as Hamedan, it is this amount is higher (23%) (19). In Tehran, Iran Klebsilla spp. showed the highest sensitivity to CP (95.1%) (16). The sensitivity of Proteus spp. to all antibiotics except CN was seen. In our survey more than 45% of E. coli isolates were resistance to three or more antibiotics. These results can be showed the unsuitable usage of antibiotics and prevalence of multi drug resistance (MDR) bacteria in Iran. MDR isolates may complicate the therapeutic management of patients with infection by increasing the morbidity and treatment costs by limiting the therapeutic options (4). The difference impacts of these antibiotics, not only in different countries but also in different regions of a country emphasizes that a public and ongoing regional surveillance on the activity of antibiotics and alternative therapies should be closely monitored to assist physicians to the facilate a safe and effective therapy for urinary tract infections.