1. Background
Toxoplasma gondii is an obligate intracellular parasite with a worldwide distribution (1). It was first discovered by Nicolle and Manceaux in 1908 (2). This microorganism has very low host specificity and will probably infect almost any mammals and birds (3). It was reported in about 20% to 90% of the adult population in different regions of the world who had contact with this parasite (4). The prevalence of this organism depends on many factors such as age, geographical area, consumption of raw or half-cooked meat and contact with cats. In Iran, the seroprevalence of this infection has been reported between 40 and 70% at different regions (5).
This parasite is a well-known opportunistic pathogen in immunocompromised subjects, including patients with HIV and organ transplant recipients (6, 7). It remains a rare but important pathogen in renal transplant recipients (8-10). Renal transplant patients must take immunosuppressive drugs to prevent rejection and decrease the immune systemic response (11, 12). By continuous administration of these drugs, they are prone to acquire many opportunistic infections such as toxoplasmosis (13, 14). Toxoplasmosis in immune-compromised patients may result from reactivation of past infection or transmission to the host from an infected donor (13, 15).
There were various clinical presentations of toxoplasmosis in renal transplant recipients. Fever (the most frequent clinical sign) (85%), (15), Neurologic symptoms such as somnolence, confusion, altered consciousness, seizures, headache, drowsiness and lethargy were also observed (16, 17). However Clinical signs alone are unable to diagnose toxoplasmosis, so confirmatory parasitological and serological evaluations are needed (18). Diagnosis and follow up of toxoplasmosis in these patients must be done in the first three months, because several studies have reported that the most infections occurred within the first three months after transplantation, which is the period of maximum immunosuppression (15).
The diagnosis of toxoplasmosis can be done by a variety of methods. The difficulty lies in determining whether the infection is acute or chronic. Acute infection can best be verified by isolating T. gondii or T. gondii DNA from the patient's blood or finding tachyzoites in tissue or bodily fluids. In Ahvaz, capital of Khuzestan province, Southwest of Iran, the evaluation of toxoplasmosis in renal transplant recipient had not been investigated, and it was the first serology and molecular survey conducted in this point of the country.
2. Objectives
The current study aimed to compare the outbreak of toxoplasmosis in renal transplant recipients with those of the control group and also to evaluate the possible dissemination of this infection in the patients.
3. Materials and Methods
3.1. Sample Collection
In this cross-sectional study a total of 100 renal transplant recipients who referred to hospitals of Ahvaz Jundishapur University of Medical Sciences, were selected as an experimental group, and 100 healthy people were selected as a control group. At first each of them filled out a questionnaire including demographic data, such as gender, age and any contacts with cat and domestic animals, consumption of under-cooked meat, educational level of the population and etc. Approximately 10 mL of venous blood was drawn at the same time and divided in two parts, first for testing the presence of Toxoplasma-specific IgG and IgM antibodies and the second for PCR technique. Serum samples were stored at -20ºC until the ELISA diagnosis test was applied.
3.2. Serology Methods
The specific IgG and IgM antibodies of T. gondii in the two groups were detected by ELISA technique (Torch-IgG, IgM-Trinity Biotech Company). According to the manufacturer’s protocol, the IgG and IgM levels lower than 1.1 UI/mL were reported as a negative and levels equal or higher than 1.1 UI/mL were reported as a positive.
3.3. DNA Extraction
DNA from blood samples was obtained by using Genomic DNA blood and tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions (Bioneer, USA). At first each blood sample was mixed with an equal volume of sterile 2% Dextran T 500 (Pharmacia) and regimented for 15 minutes. The leucocyte-rich supernatant was collected in 2 ml tubes and centrifuged at 2000 x g for 10 minutes. The pellet was kept at -80°C for PCR (19).
3.4. PCR Amplification
PCR was done to amplify the coding region of the GRA6 gene. Target of the GRA6 gene was amplified with specific primers, forward primer, 5'-GTAGCGTGCTTGTTGGCGAC-3' and reverse primer, 5'-ACAAGACATAGAGTGCCCC-3' (20). Amplification was carried out with 10 pmol of each primer and 10 µl of extracted DNA in 20 ml of modified Taq DNA polymerase Master Mix RED reaction (Bioneer Korea) containing 75 mM Tris-HCl (PH 8.5), 20 mM (NH4)2SO4, 1.5 mM MgCl2, 0.1% Tween 20, 0.2 mM dNTPs, 0.025 U/µL Amplicon Taq DNA polymerase, inert red dye and a stabilizer. The PCR conditions were 5 minutes at 95°C followed by 35 cycles of 30 seconds at 94°C, 1 minute at 60°C, 2 minutes at 72°C, and a final elongation of 72°C for 7 minutes (20). The PCR products were detected in 1.5% ethidium-bromide-stained agarose gels.
3.5. Statistical Analysis
Data were analyzed by using SPSS (version 15) software. Significance of difference was analyzed by chi-squared test.
4. Results
Totally 34 cases (34%) of the renal transplant recipients and 26 cases (26%) of the control were positive for anti-Toxoplasma IgG. In addition, 18 from renal transplant recipients (18%) and 4 from control (4%) were positive for anti-Toxoplasma IgM. In the current study there was no significant association between consumption of under-cooked meat and close contact with domestic animals with seropositivity of toxoplasmosis (P > 0.05). The difference between female and male, urban and rural subjects was not statistically significant (P > 0.05). There was no significant difference between consumption of pipeline water source and unfiltered water in prevalence of Toxoplasmosis (P > 0.05). The influence of educational level on seropositivity rate of Toxoplasma antibody was not observed. The frequency of serum anti-Toxoplasma IgG and IgM in renal transplant recipients and members of the control group according to age and sex are shown in Tables 1 and 2.
aAbbreviations: RTRG, Renal Transplant Recipients Group; CG, Control Group
aAbbreviations: RTRG, Renal Transplant Recipients Group; CG, Control Group
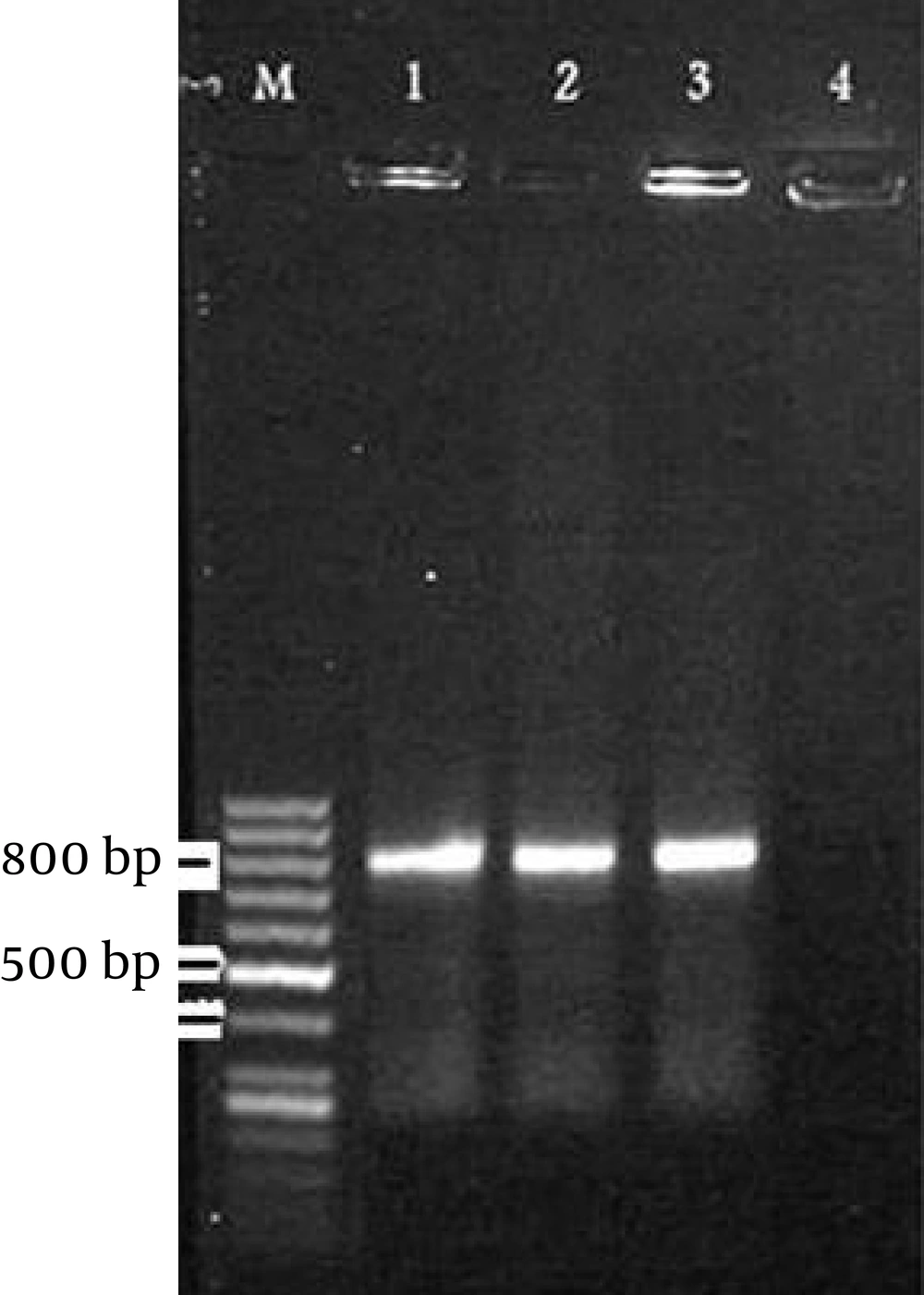
PCR had positive results with two of the 100 blood samples from renal transplant recipients group but there were no positive results with those of the control group samples. The size of the PCR products was around 800 base pair (bp) (20) (Figure 1).
5. Discussion
Over half of the world’s human population is estimated to carry a Toxoplasma infection (21). Transmission to humans usually takes place by eating raw meat containing tissue cysts or eating infectious oocysts via food or water contaminated with feline faeces. It is rare but potentially life-threatening in patients with immune-depression, such as transplant patients (22). In these patients, transmission of T. gondii from a seropositive donor to a seronegative recipient is a significant potential cause of the disease (20, 21). Toxoplasmosis in renal transplantation can cause mortality in up to 65% of recipients (15). In Iran, renal transplant donors and recipients are not screened for toxoplasmosis since T. gondii infections in renal transplant recipients are thought to be rare.
In many studies, contaminated drinking water, close contact with cats as domestic pets and eating uncooked meat have been implicated as sources of Toxoplasma infection in humans worldwide (23-27), but there was no statistically significant association between these risk factors and Toxoplasma seropostivity in the current study. In this study specific IgG and IgM antibodies of T. gondii in the experimental and control groups were detected by ELISA technique. This method is the most common approach used to identify toxoplasmosis and can be used to diagnose a primary infection (28).
In addition, PCR was used to detect T. gondii parasite in blood samples, because serological methods are usually not useful to distinguish recent from past infections and T. gondii-specific IgM remains detectable and may remain detectable for more than 1 year after primary infection (29). Especially in immunodeficient patients antibody titers are much more difficult to interpret and in the case of reactivation, the value of serology is limited. PCR method is a sensitive and specific tool to detect T. gondii that can prove the presence of Toxoplasma in the samples (30-32). In the current study, the frequencies of the two antibodies (IgM and IgG) in renal transplant recipients were significantly higher than those of the control group, especially for IgM.
Gharavi et al. also reported a significantly higher prevalence of anti-T. gondii IgM and IgG antibodies in renal transplant recipients than those of the control subjects (33). Gharavi et al. detected IgM and IgG anti-Toxoplasma antibodies in renal transplant recipients before and after transplantation by different methods and detected no IgM positive reactions in pre-transplantation samples. However, IgM was detected in post-transplantation samples (34). Nateghirostami et al. also identified the IgG antibody titer against Toxoplasma in renal transplant recipients by IFA technique. In their study the prevalence rate of toxoplasmosis was 7.1% (35). This result is significantly lower than that of the current finding (P < 0.05). This difference may be due to the methods employed, ELISA was used in the current study.
Garedaghi et al. in a cross-sectional survey on a number of 96 blood samples of the kidney recipients and a number of 96 blood samples of control group, tested by ELISA method, showed the prevalence of anti-Toxoplasma IgG in kidney recipients was 36.46% in comparison with 2.08% in control. This result is approximately similar to the finding of the current study (36). Nissapatorn et al. in a study in Kuala Lumpur, Malaysia conducted on 247 of renal patients; detected anti-Toxoplasma antibodies in 56.7% (140/247) of the patients. They indicated valuable differences between renal patients and control groups (37). The current study detected the T. gondii parasite in 2 of the blood samples. All PCR-positive samples belonged to patients with positive for IgM anti-Toxoplasma antibodies.
Lamoril et al. detected T. gondii in 19 blood samples of patients with AIDS by PCR (19). Aubert et al. detected T. gondii in peripheral blood samples, 30 days after renal transplantation by PCR in 1996 (38). PCR techniques are suitable for immune-compromised patients because these methods do not depend on the host immune responses and allow for direct detection of T. gondii DNA from a variety of clinical samples and facilitate the diagnosis and follow-up of the severe infection in patients with immunodeficiency (39, 40).
In the current investigation the presence of Toxoplasma parasite was detected successfully in IgM positive patients indicating dissemination of the parasites in this group and no amplification was seen with DNA from blood samples of IgM negative patients and controls. This study revealed higher prevalence of toxoplasmosis in renal transplant recipients compared to healthy people and also indicated dissemination of toxoplasmosis in the patients. Toxoplasmosis in renal transplant recipients is a complication that may cause important morbidity and mortality. Physicians must be aware of this possibility and should apply preventive measures and make an early diagnosis in the case of compatible symptoms. Screening and follow up for toxoplasmosis in this vulnerable group are suggested.