1. Background
Among all microorganisms, spore-forming Bacillus strains are found in various natural areas, are resistant to various environments and ecosystem, easy to isolate, have different physiological abilities and produce valuable biological products. Due to their endospores, they are resistance to UV, heat, pH and their hydrolysis activity can be used in different industries (1-3). Bacillus sp. is gram positive, endospore forming and straight rods developing that forms individual endospore in each cell against extreme conditions. In these bacteria, presence of oxygen cannot prevent the sporulation phase.
One group of significant biological substances that are produced by the genus Bacillus are alkaline proteases with several important applications in the daily life and common industries such as food detergent, alcohol and beer production medical and cosmetic/sanitary and leather, beside wastewater treatment, biotransformation, hydrolyzed proteins, oil manufacturing (2-4). Bacillus sp. produces tremendous hydrolysing enzymes out of waste materials. Among all these enzymes, alkaline protease is a very important product which several industries have been involved in its production and marketing all around the world (1, 3, 4). Bacillus sp. is detected and isolated using 80°C water bath which kills all the vegetative bacteria and resistant forms except Bacilli and Clostridia endospores.
An alkaline protease has been produced by Bacillus cereus MCM B-326, using a medium containing soya flour, starch and wheat bran as the main components. The produced enzyme has been applied for the dehairing of buffalo hide (5). Several agro-industrial waste substances have been used to produce alkaline protease from an alkalophilic Bacillus sp., among which the green gram husk resulted in the highest production of the enzyme, using solid-state fermentation (6). Feather is resistant to protease hydrolysis due to the existence of different bonds in its structure but some microorganisms are capable of its hydrolysis (7, 8). Although keratin is a poultry byproduct, it can be transformed to an animal food source, in addition to reducing the environmental pollution (8, 9). Different microorganism such as Actinomycetes, fungi and yeast strains as well as many types of bacteria can produce feather-hydrolyzing keratinase (10-14). These enzymes are important and numerously applied in different industries (15).
2. Objectives
The aim of this study was to investigate and evaluate the alkaline keratinase production by a nitrogen fixing, UV-stable bacteriocin and IAA producer Bacillus sp. (16). Revealing the prospective multi-area application of B pumilus, this research might lead to cell biomass production from the waste chicken feather.
3. Materials and Methods
3.1. Microbiological Culture Media
The composition of basal mineral medium, which has been used in this study, was [g/L]: Glucose [1], (NH4)2SO4 [1], KH2PO4 [0.7], K2HPO4 [1.4] and MgSO4.7H2O [0.1]. The pH was adjusted to 7.4, Medium was sterilized for 15 minutes at 121°C upon 15 lbs. L-tyrosine, Gelatin, Caseine powder and poultry feather were substituted with glucose when enzyme activities were being studied (17).
3.2. Screening, Isolation, Identification and Characterization of the Best Alkaline Protease Producing Bacillus sp.
Different nitrogen-fixing Bacillus isolates were obtained from the sea water of North of Iran and Persian Gulf, using heat-shock and incubation in an anaerobic jar, based on the method used by Shokri and Emtiazi (18). Identification of the isolates was initially carried out on the basis of their morphological, cultural, and biochemical characteristics using standard methods (19) and then confirmed using 16S rRNA. The related PCR reactions were performed using DNA extracts with universal primers RW01 (5'-AAC TGG AGG AAG GTG GGG AT-3') and DG74 (5'-AGG AGG TGA TCC AAC CGC A-3') obtained from CinnaGene Company (Tehran, Iran).
3.3. Evaluation of Alkaline Protease
Protease-producing Bacilli were enriched on feather with alkaline pH, 9, 10 and 11 and the pH of the culture media was optimized with 1 M NaOH. The best alkaline protease producers were screened by measuring the released protein OD at 595 nm. The alkaline protease activity evaluation was confirmed using alkaline skim milk, Gelatin and Casein agar culture media with pH 8, 9 and 10 (2).The maximum crude enzyme production was optimized at different pH, salt and substrate levels.
3.4. Assay of Alkaline Protease
In all the experiments, alkaline protease assay was based on extra protein production from feather in different pH levels. As the supernatant enzyme, protease activity was assessed according to soluble protein production on feather, which was measured using Bradford reagent at 595 nm. The calibration line of serum bovine was obtained for Bradford method (20).
3.5. Crud Keratinase Freezing
B. pumilus was grown on feather as the only source of carbon, nitrogen and energy for 3 days. The cells were separated with centrifugation at 6000 rpm and the supernatant was condensed with freeze drying. The concentrated enzyme was assessed using 12% Acrylamid gel SDS-PAGE.
3.6. SDS-PAGE and Zymogram of Protease
To estimate the extracellular protein obtained from the keratin-grown cells, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used. After electrophoresis, the gel, containing the protein standard ladder (low-molecular-mass range marker protein standards; Sigma, St. Louis, MO), was stained with Coomassie Brilliant Blue (CBB) R-250.
Zymography method has been proposed by Yamada et al. following Heussen and Dowdle. It is a method which can detect the protease activity in gel. This method was followed by 7.5% mini-gel SDS-PAGE. After electrophoresis, the gels were divided in two parts. A part was washed in 2.5% (v/v) Triton X-100 for 30 minutes to remove SDS and allow the renaturation of proteases. The gels were then incubated in phosphate buffer for 15 hours at 37°C and stained with Coomassie brilliant blue (CBB) R-250. Another part was transferred into 1% casein-solute and incubated for 35 minutes at 37°C in the presence of 50 mM tris buffer with pH of 8.5. Following incubation, the gel was stained with Coomassie Brilliant Blue G-250 to visualize the hydrolysis zones (21).
4. Results
4.1. Screening the Proteolytic Strain Among Nitrogen Fixing Bacteria
Several nitrogen fixing bacilli were isolated by Zaghian et al. (16) these strains were enriched in different protein media to screen the best proteolytic Bacillus. The proteolytic activities of these strains were assayed using skim milk agar and casein agar. By this approach, presence of a clear zone was suggestive for proteases production from the test bacterial strains. The enriched strain on the feather was identified as B. pumilus with 99% identity according to partial 16S rRNA gene sequence and it was given the strain designation of ZED17 (with accession No. JNO54254.1). This strain which was identified by biochemical and 16S rRNA with a high protease capability was selected for further studies.
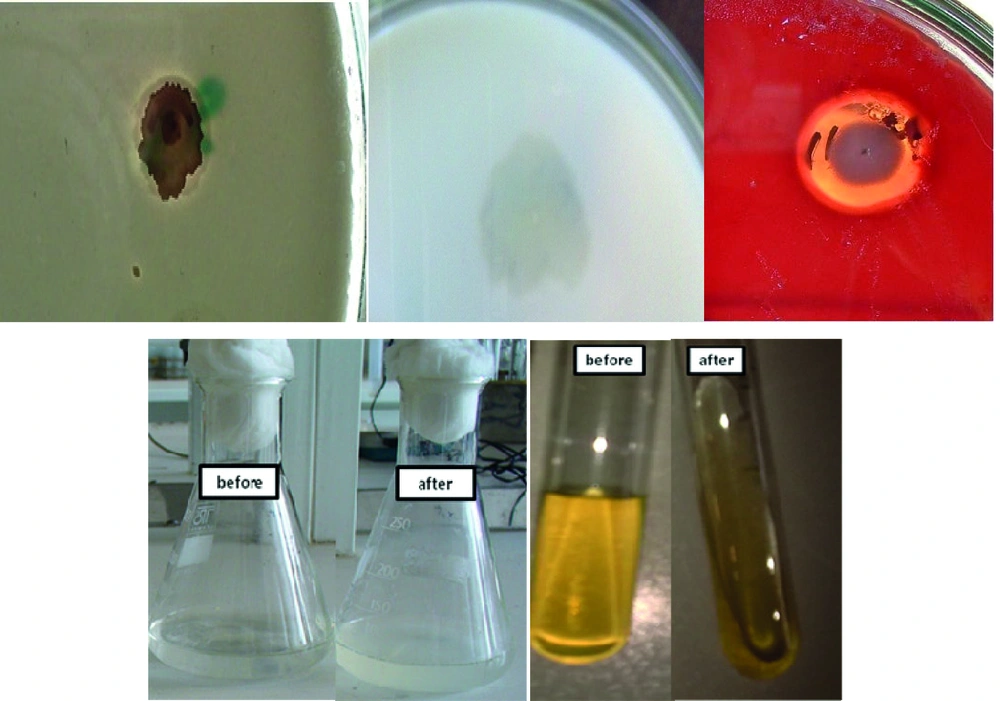
The mentioned strain has high hydrolytic activities such as Gelatinase, Protease, Caseinase, Keratinase, Phosphatase and hemolyse activities which are shown in Figure 1. Even when this strain was only grown on feather, the multi-protease activity was determined in the supernatant.
4.2. Optimization of Keratinase Activity
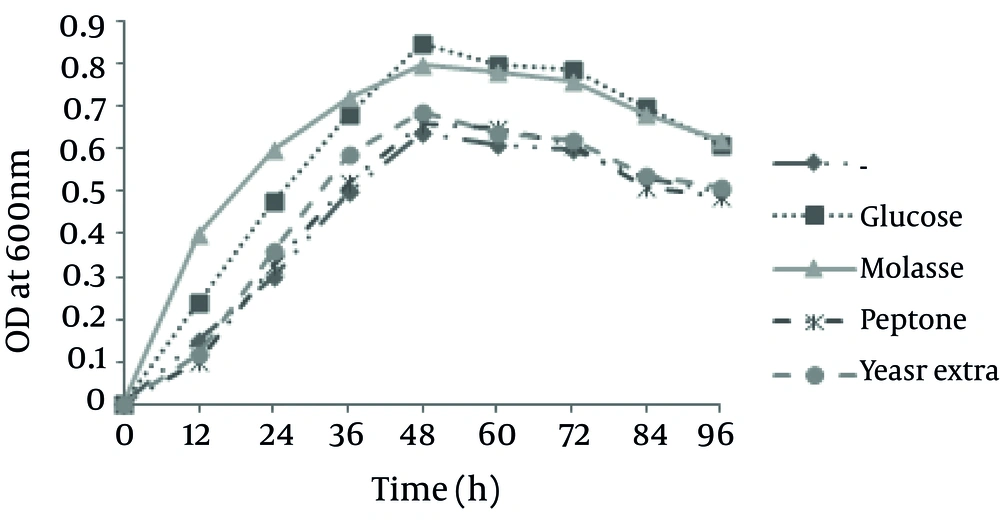
Enzyme formation is largely dependent on the condition of culture growth and composition of nutrient medium. Various carbon and nitrogen sources were used for production of protease by B. pumilus ZED17. The growth rate of this strain on keratin has been studied and the results are presented in Figure 2. As it is obvious, the maximum growth on 10 g/L feather is occurred after 48 hours in which the maximum optical density was 0.6. However, adding another carbon or nitrogen source increases the maximum growth to 0.8 in 600 nm.
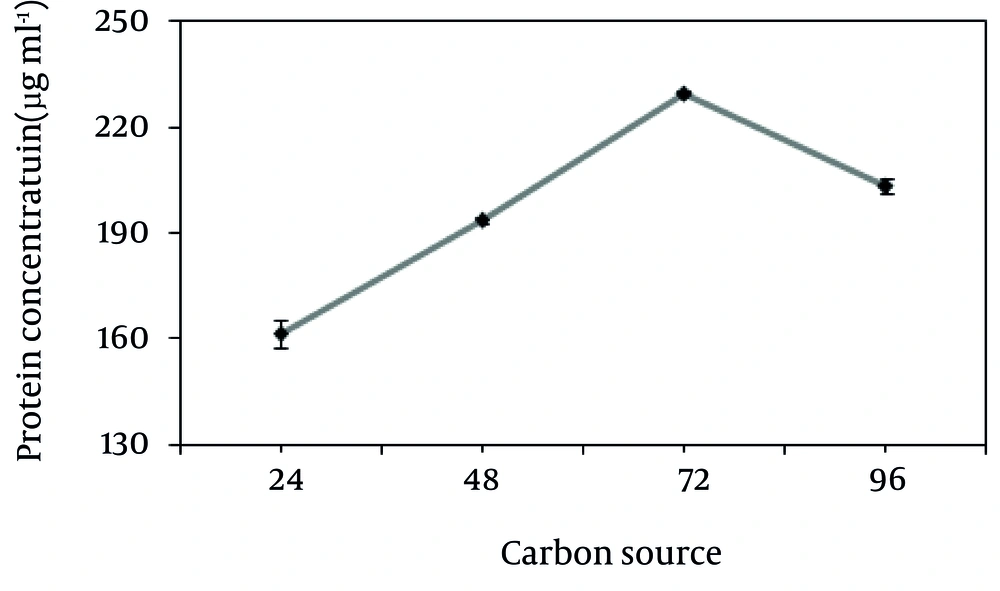
The protein production rate of this Bacillus on keratin has been studied and the results are given in Figure 3. The maximum production of protein on 10 g/L feather and 1 g/L glucose as the carbon source occurred at the third day of incubation.
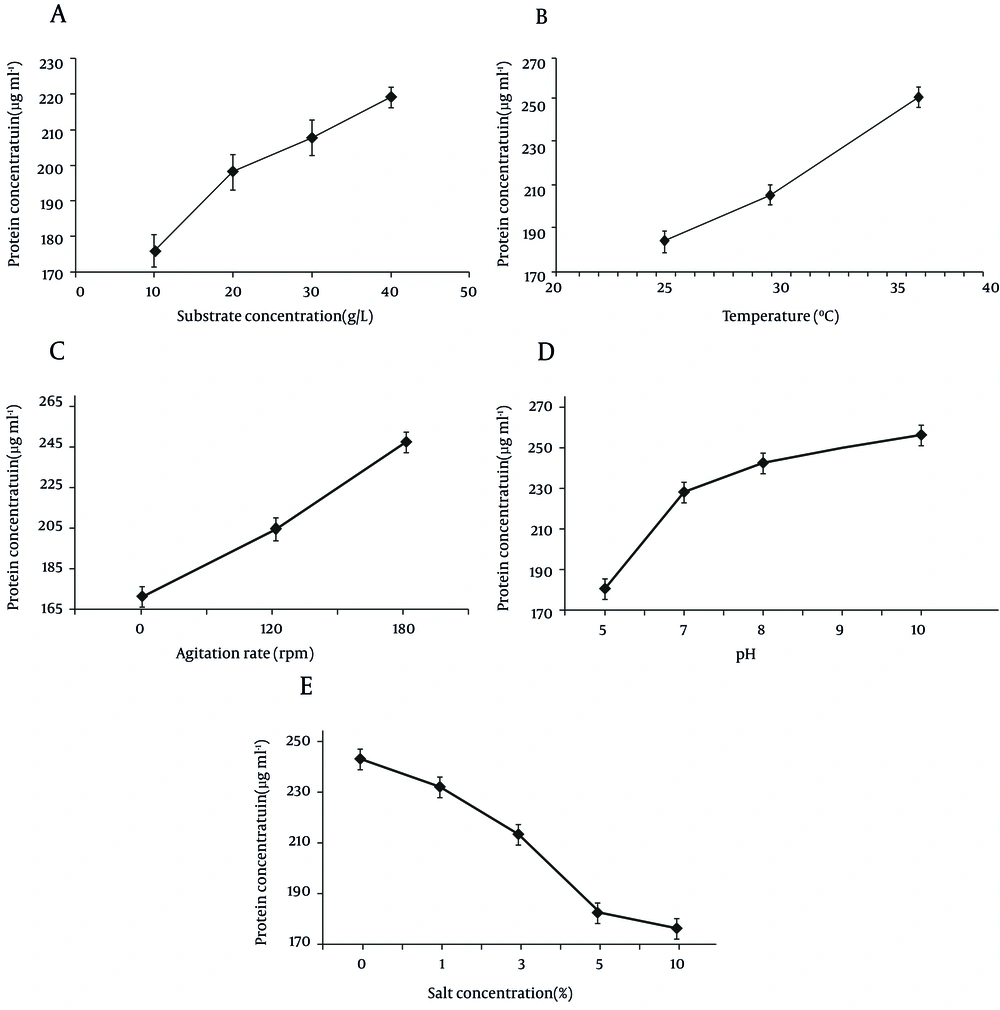
The protease production was higher when glucose was added to the keratin basal medium, in comparison with other sources (Figure 2). As shown in Figure 3, the maximum protease production occurs 72 hours after the incubation in the stationary phase. The effects of keratin concentration, addition of sugar, temperature, agitation and NaCl on protein production from chicken feather by this strain were investigated and the results are presented in Figure 4. Protease activity of Bacilllus was stable under heat and acidic/basic treats and the optimum pH and temperature for keratinase production were 10 and 37ºC, respectively.
As shown in Figure 4, this enzyme is alkalophyle; so 40 g/L of keratin as the only source of substrate at pH 10 has an increasing effect on protein production from feather.
4.3. Enzyme Activities
The supernatant of B. pumilus ZED17 that has been grown on feather was used for keratinase activity detection in different conditions. The results showed that a high activity of keratinase was obtained from B. pumilus ZED17 on feather meal which was assayed at 40°C in alkaline pH and 0.1 M NaCl. The supernatant of keratin-grown cells had protease activity which supported the high proteolysis activity of this isolate on keratin.
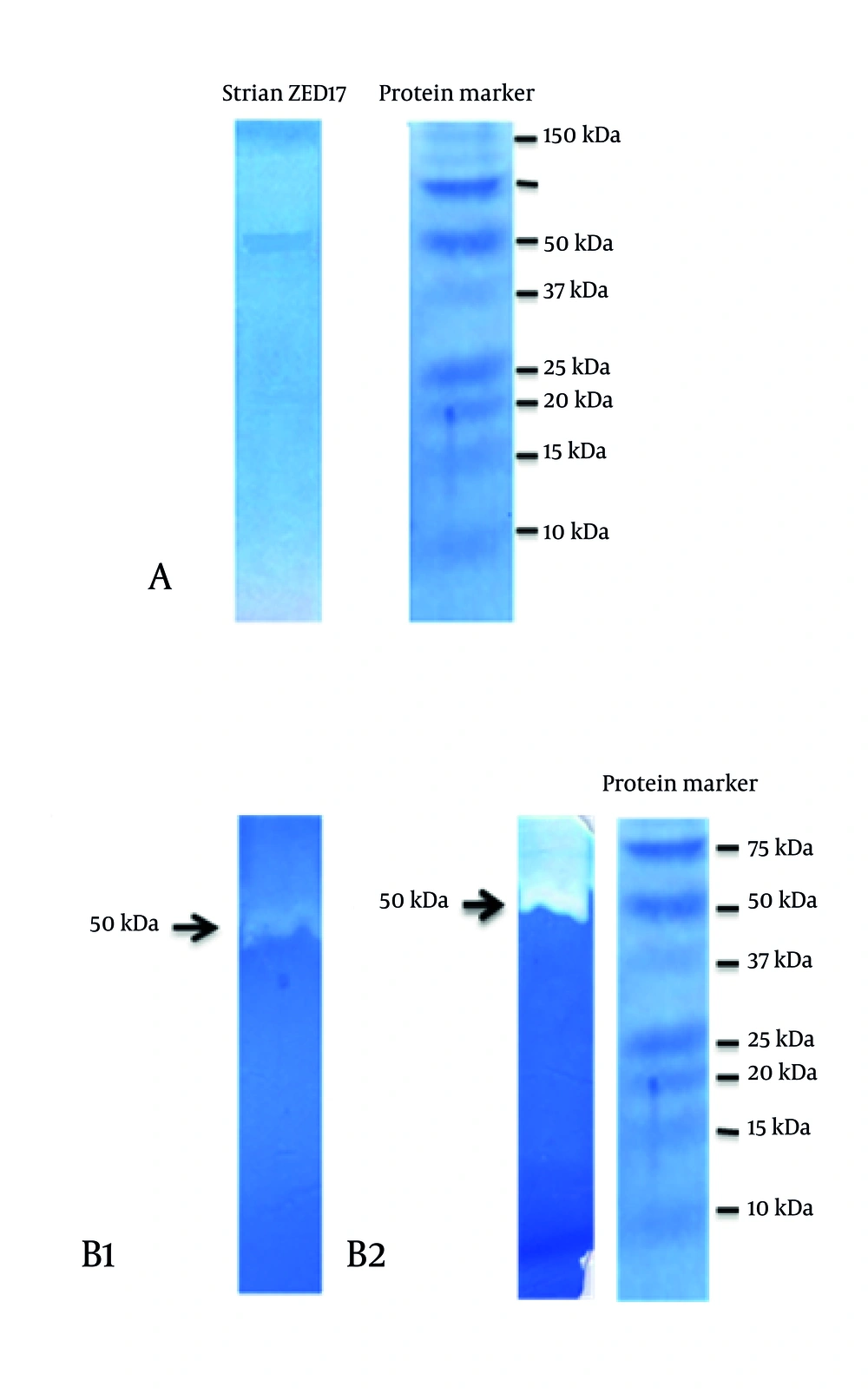
4.4. SDS-PAGE and Gelatinase Page of B. pumilus ZED17
The current concentrated enzyme was run for electrophoresis on 12% (w/v) SDS-PAGE and a single band was observed. Using standard protein markers, size of the partially-purified enzyme on zymograph gel with clear area was found to be about 50 kDa (Figure 5).
5. Discussion
B. pumilus ZED17 has different activities such as Gelatinase, Protease, Caseinase, Keratinase, Phosphatase and Cytolysis. Maximum production of protein on 10 g/L feather and 1g/L glucose, as the carbon source, occurred at the third days of incubation. The optimum pH and temperature for enzyme production were 10 and 37ºC, respectively. The results showed a higher activity of keratinase obtained from B. pumilus ZED17 on feather meal, which was assayed at 40°C in alkaline pH and 0.1 M NaCl.
Protease activities of Bacillus vary with pH alternation, which have been reported by Guangrong and others (22-24). Among the proteases, alkaline proteases are the most important ones in the industry and usually, maximum production occurs in the stationery or post exponential phase of their growth. The compositions of medium, carbon sources and other factors are important for protease production in Bacillus. Morya et al. (24, 25) reported that optimum temperature for Bacillus sp. Protease action is in the range of 30-70ºC, while, Ammar et al. (26) obtained 55ºC as the optimum temperature. Some other researcher have come up with rather similar results, e. g. 60ºC at pH of 8 for B. firmus and 60ºC at pH 9 for Bacillus strain SAL1 (27, 28).
A variety of molecular weights for proteases produced by other Bacillus species has been reported: 49 kDa Bacillus sp. HUTBS71 (29), 75 KDa Bacillus sp. S17110 (30), 30.9 kDa Bacillus sp. HS08A (24) and 75.0 kDa Bacillus sp. S17110 (31). Bacillus strain SAL1 has shown alkalin protease band with the molecular weight of 27 kDa in the SDS-page (32). Fakhfakh-Zouari (31) isolated and characterized a keratinolytic enzyme from a newly isolated B. pumilus strain A1 on chicken feather meal with molecular mass of 34000 Da. They also found that the optimum condition for keratinase production on keratin was in pH 9.0 at 60ºC. Novel Keratinase from B. subtilis S14, exhibiting remarkable dehairing capabilities, has been used as an alternative for sodium sulfide (17). Mazotto et al. described that B. subtilis 1273 uses feather as a substrate for keratinase production (32). A 45-kDa keratinase obtained from B. pumilus KS12 was reported by Rajput et al. (33).
In the present study, , size of the concentrated enzyme was found to be about 50 kDa using standard protein marker and due to its multi-activity on different substrates such as Casein, Gelatin and keratin, it was introduced as a multi-enzyme. The zymogram test showed hydrolysis activities of casein around the 50-kD band.