1. Background
The success of endodontic treatment is directly influenced by elimination of microorganisms in root canals. Effective antimicrobial irrigations are too significant during root canal preparation, because they aid in cleaning the root canal, lubricating the files, and flushing out debris (1, 2). Ideal irrigants should have strong antimicrobial effect but nontoxic to the periapical tissues when they run over from the apex (3). It is difficult to eliminate all the microorganisms and throw out the organic debris from the root canal system regardless of the irrigant and instrumentation (4, 5). Generally in endodontics, 5.25% NaOCl was superior in its antimicrobial abilities compared with the other used irrigants (6). The concentration and toxicity of NaOCl is directly proportional (7). Using low concentrations of NaOCl not only reduce cytotoxic and irritating properties, but also decrease antibacterial effects (8).
Recently, ozone has become one of the most important disinfecting agents used in dentistry. It can be administered in either gaseous or aqueous form. Both of them may act like powerful antimicrobial agents that are strong and fast oxidizers of cell walls and cytoplasmatic membranes of microorganisms. For these reasons, ozone is considered as one of the best bactericidal, antiviral, and antifungal agents (9, 10). The gaseous form of ozone is a respiratory irritant, and can also cause dryness in the mouth and throat, headache, chest restriction, and coughing (11). Besides, the antimicrobial efficacy of gaseous ozone has been emphasized in some studies. For instance, in a research, gaseous ozone/oxygen mixture with a low dose completely inhibited the growth of all potentially pathogenic bacterial strains that cultivatein Petri dishes (12).The other studies that also determined the gaseous ozone effect against microorganisms in the root canals, complete elimination of bacteria could not be achieved (13-15).
Aqueous ozone essentially shows no toxicity to oral cells as in vitro (10). Because it is highly unstable, aqueous ozone should be used as soon as possible after being mixed. It is very difficult to keep this mixture at the same concentration for a long time (16). Many studies demonstrated the antimicrobial effect of aqueous ozone, emphasized insufficient disinfectant effect of aqueous ozone against pathogenic microorganisms in dental plaque (10), root canals (13) and acrylic resin plates (17). Due to the these properties, ozone may be used as a new alternative disinfectant agent in root canals but there is also the need to investigate whether it has the same effect like NaOCl or not.
2. Objectives
The current study aimed to investigate the antifungal effects of aqueous ozone administered via manual or ultrasonic irrigation techniques and gaseous ozone against Candida albicans in root canals. Moreover, data were compared with antifungal effects of saline (positive control) and NaOCl (negative control) groups for the disinfection of root canals infected by C. albicans.
3. Materials and Methods
3.1. Sample Preparation
For orthodontic or periodontal reasons, in the present study, 50 single-rooted single-canal human mandibular permanent premolar teeth that were freshly extracted without caries and restorations, were used. Digital radiographs of teeth were taken from buccal and approximal directions to determine the number and morphology of canals. Informed consent was obtained from the patients before starting the research, and the study was approved by the Local Ethics Committee on Human Research of Cumhuriyet University.
Having been cleaned of residues, freshly extracted teeth were kept at +4°C and 0.9% saline solution until the study was applied. Below the level of enamel-cementum junction, the coronal portions of the teeth were cut using sterile diamond discs under cooling water to obtain a 14-16 mm length of each root. Then the root canals were entered with #15 K-File (Mani Inc, Tochigi, Japan) hand tools, and the path of canal was determined. The tip of the file was used to measure the length of each canal until it became visible in the apical foramen. Then it was withdrawn 1 mm from the measured length. The root canals were shaped with ProTaper (Dentsply, Tulsa Endodontics, OK, USA) rotary Ni-Ti instruments using the crown-down method by the electric motor (Denta ports DP-ZX, J. Morita MFG, CORP, Kyoto, Japan). Firstly, the coronal third of the roots were expanded with SX files. Secondly, the median third of roots were reached with S1 and S2 files. The F1, F2, and F3 files were applied, respectively, to shape the apical third of the canals. The canals were irrigated with 1 ml of 5.25% solution of NaOCl after the usage of each file.
The roots were irrigated with 17% EDTA, 5.25% NaOCl, and distilled water, respectively, for 10 min to remove the smear layer formed during root canal preparation and then were dried with paper point. The bottles were placed in an autoclave to ensure sterilization for 20 min at 121°C (Melag, Euroklav 23V-S, Germany). Then 3-fold nail polish (L'Oreal Jet-Set Diamond, Paris, France) was applied to whole root surfaces of the teeth, including the root tips. Rubber caps embedded in the teeth were sterilized by Ethylene oxide and then placed in bottles.
3.2. Microbiologic Procedures
C. albicans (ATCC 10231) strains were cultured in the liquid nutrient media brain heart infusion broth and were incubated at 37°C for 24 h. Prior to the each experiment, 0.5 McFarland turbidity was set with kristalspec TM, and McFarland standard number 0.5 was used to improve broth to obtain fungal growth in the amount of 1.5 X 104 colony forming unit (CFU/mL). Then 10 μl of the yeast culture was transferred to the mechanically expanded lumen of the root canal using a sterile micropipette and then kept at 37°C for 24 h. The sterile paper points (Dentsply, Maillefer) were placed in the root canals inoculated with yeast to control fungal growth. Paper points were left for 5 min within the root canal, soaked with the broth. Then paper points were put into sterile eppendorf tubes containing 0.5 ml brain heart infusion broth (Merck 1.13825). After waiting for 15 min, 50 μl of liquid medium was taken with a sterile micropipette from the Eppendorf with mixed vortex and was smear-planted to solid medium (Sabouraud dextrose agar), which was split before and after the disinfection applications.
3.3. Experimental Groups
Group 1, Saline (positive control) group: Infected root canals were irrigated for 300 s with 0.9% saline solution.
Group 2, NaOCl (negative control) group: Infected root canals were irrigated for 300 s with 5.25% NaOCl.
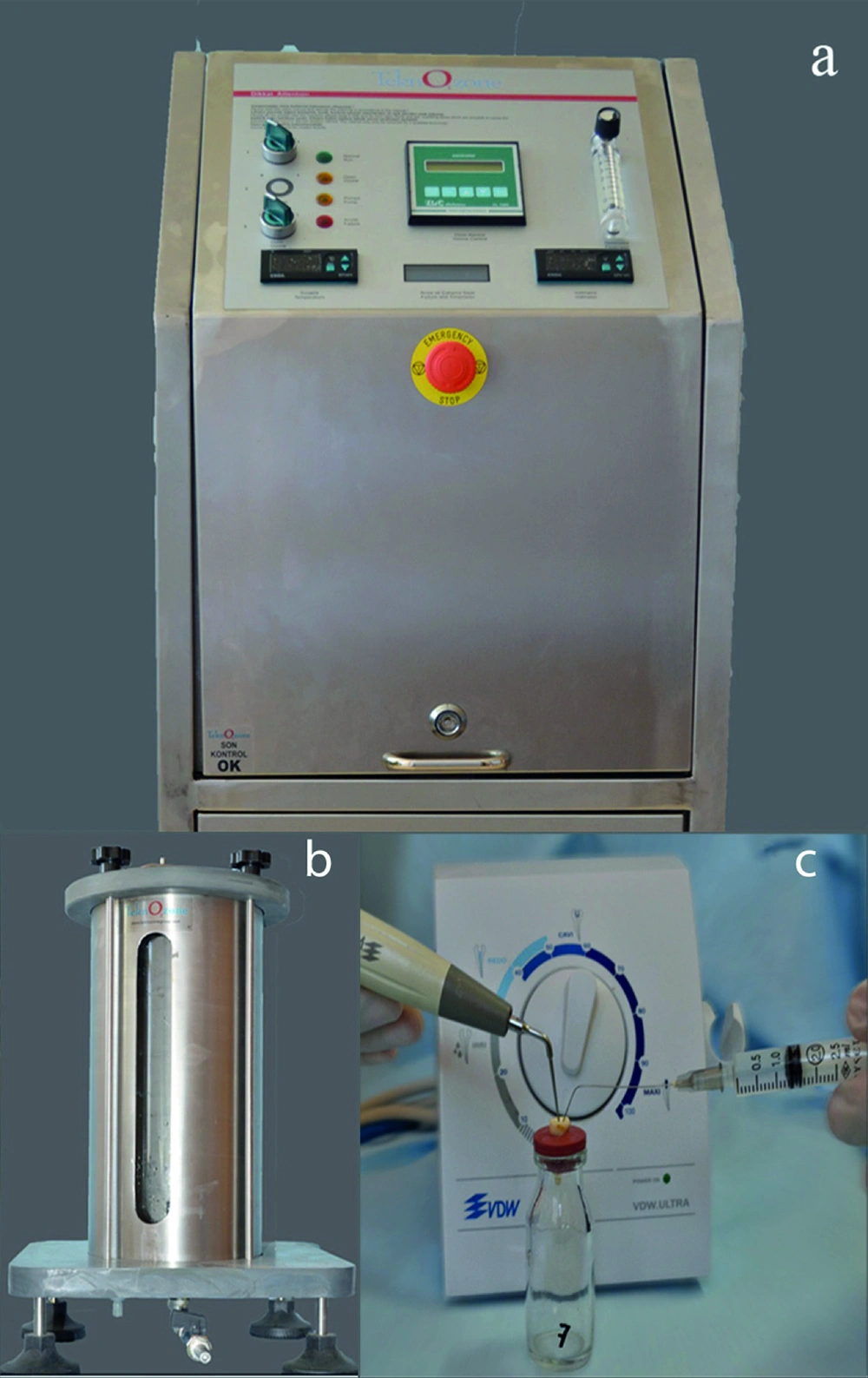
Group 3, Aqueous ozone with manual technique group: Aqueous ozone was obtained from the custom-made ozone generator (TeknO3zone, Izmir/Turkey) by the TeknO3zone company (Figure 1a). The ozone density of the distilled water was measured with the probe in the reactor tank (Figure 1b) and was shown by the digital indicator on the generator. Infected root canals were irrigated for 300 s with 4 mg/L aqueous ozone (TeknO3zone, Izmir/Turkey). Control of power was maintained automatically by the automatic balancing system.
Group 4, Aqueous ozone with ultrasonic technique group: Aqueous ozone was obtained from the custom-made ozone generator (TeknO3zone, Izmir/Turkey) by the TeknO3zone company. Infected root canals were irrigated with 4 mg/L aqueous ozone and simultaneously combinated with ultrasonication that was obtained from VDW. ULTRA (Satelec, Merignac Cedex, France) (Figure 1c) device for 300 s.
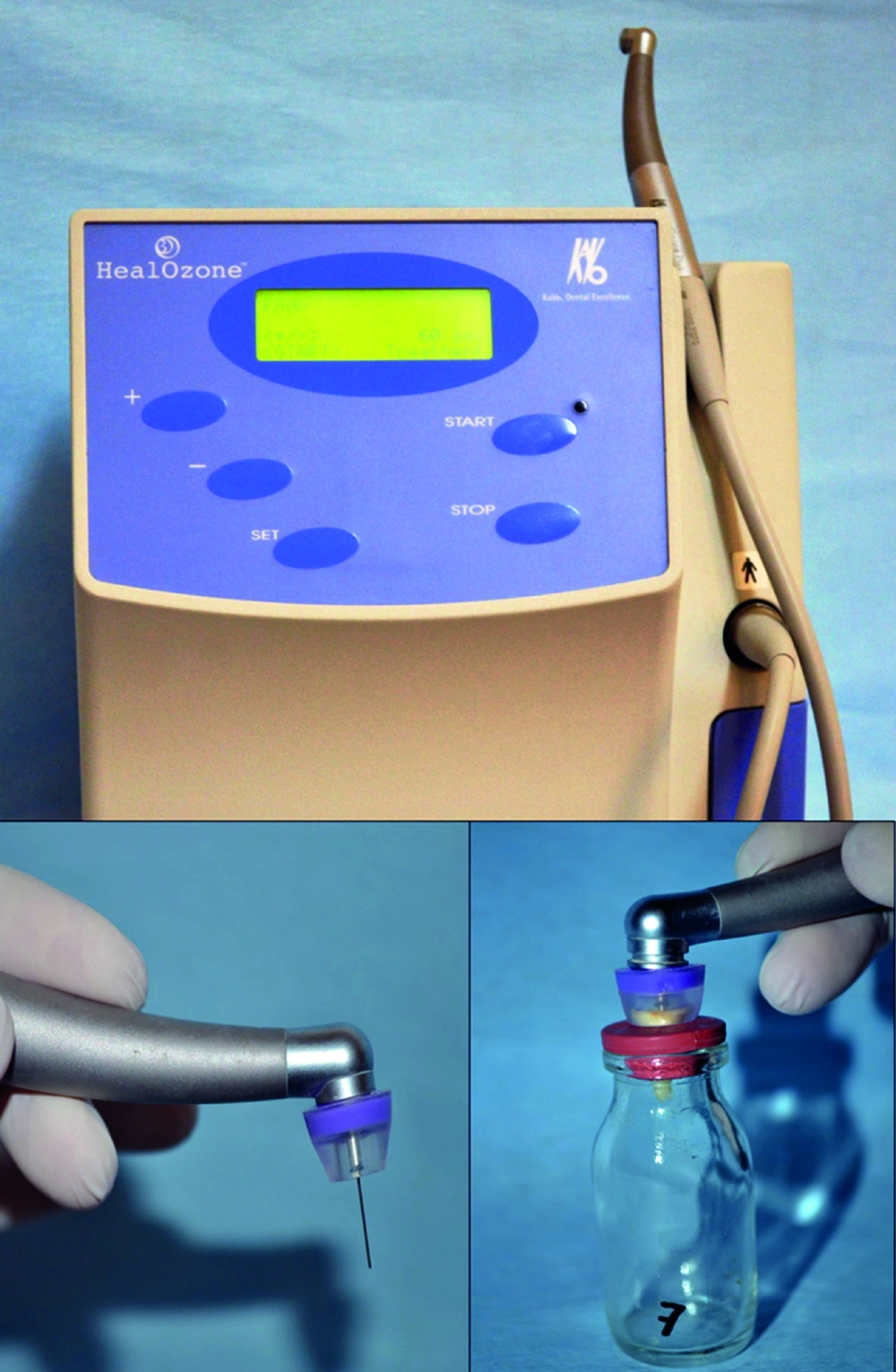
Group 5, Gaseous ozone group: Gaseous ozone was obtained from an ozone generator (Heal Ozone, KaVo, Germany) by vacuum to the root canals for 300 s with a hand piece on which was fixed 5 mm diameter silicone caps and endodontic cannulas (Figure 2).
3.4. Antifungal Evaluation
Root canals were contaminated and then waited for 24 h. Paper points were placed and waited for five min in root canals before the application of irrigation to control the growth of microorganisms. They east counting of samples was done to ensure standardization and the CFU values of samples, under 1x104 CFU/mL, were excluded. After the application of irrigation, CFU counting of the breeding colonies of microorganisms were performed in Sabouraud dextrose agar. Then log CFU values were calculated.
3.5. Statistical Analysis
SPSS statistical software program (14.0 Version, SPSS Inc., Chicago, USA) was employed to analyze the variation data of irrigation solutions. The data were subject to statistical analysis among five different irrigation solutions using one-way analysis of variance (ANOVA). When significant differences were observed, Tukey post hoc test was applied to examine pair wise differences at a significance level of 0.05.
4. Result
Mean, standard deviation, and median values obtained after application of irrigation are shown in Table 1. Statistical comparison of log CFU mean values are shown in Table 1.
| Groups a | Mean (SD), Log CFU mL-1 | Median, Log CFU mL-1 |
|---|---|---|
| Group 1 b, Saline (Positive Control) | 2.700 (0.483) | 3 |
| Group 2 b, NaOCl (Negative Control) | 0.000 (0.000) | 0 |
| Group 3 b, Aqueous Ozone With Manuel Technique | 0.300 (0.105) | 0 |
| Group 4 b, Aqueous Ozone With Ultrasonic Technique | 0.000 (0.000) | 0 |
| Group 5 , Gaseous Ozone b, Gaseous Ozone | 1.100 (0.876) | 1 |
aCFU, colony-forming units; By the one way ANOVA, F = 44.868; P = 0.000 (P < 0.05).
bValues with same superscript letter are statistically different at P < 0.05 by Tukey’s Test.
The current study investigated the antifungal activities of disinfectant agents applied to the root canals, infected by C. albicans. There were a statistically significant differences between the gaseous ozone group and NaOCl (negative control) group (P < 0.05). Additionally, although no statistically significant differences were found among aqueous ozone groups (manual and ultrasonic techniques) and the NaOCl group (P > 0.05), few fungi were found in 2 of 10 examples in the aqueous ozone with manual technique group. There was statistically significant difference among the saline group and all other groups (P < 0.05). As a result, when gaseous ozone was used alone in root canals, its antifungal effect was insufficient. However, applying aqueous ozone with ultrasonic technique showed stronger antifungal effects than aqueous ozone with manual technique, in root canals.
5. Discussion
In endodontic treatments, one of the most important purposes is the removal of pulp and dentine debris. Cleaning the root canal system is done by chemo mechanic preparation in which the mechanical preparation and irrigation are applied together. For this reason, to achieve ideal treatment, the properties of irrigation solutions are highly important for the root canal system. Today, the most commonly used irrigation solution in endodontic treatments is sodium hypochlorite (18). Although it dissolves necrotic tissue and eliminates microorganisms effectively in high concentrations, this solution is very cytotoxic and harmful to living oral tissues. Consequently, current endodontic treatments should be effective and with virtually no side effects for disinfection of the root canal (18-20).
Fidalgo et al., (21) evaluated the antimicrobial activity of different root canal irrigants against C. albicans. The researcher found a slight reduction of the number of C. albicans after irrigation of NaOCl with 2.5% concentration. Furthermore, they observed a complete elimination of C. albicans following irrigation with 5.25% NaOCl. Similarly, in the present study, 10 ml NaOCl at a 5.25% concentration was used for 300 s to ensure complete disinfection of the root canals. This concentration of NaOCl completely eliminated the C. albicans. Consequently, NaOCl as an irrigation solution showed strong antifungal effect. HealOzone or OziCure generators are frequently used to obtain gaseous ozone. The aspirator on the generator should absorb the ozone back into the environment.
In the HealOzone generator, gaseous ozone is absorbed and then converted into the gaseous oxygen before releasing the ozone into the environment. In case of gas leakage, the system automatically stops the application of gaseous ozone. When the OziCure generator is used without sufficient absorption, ozone gas levels can reach undesirable levels. For these reasons, is safer to use the HealOzone generator rather than the OziCure generator (22, 23).
Huth et al. (13) investigated the antimicrobial efficacy of gaseous and aqueous ozone against C. albicans. Most of C. albicans were eliminated after application of gaseous ozone for 60 s. Moreover, when aqueous ozone application was conducted at high concentration (20 mg/L) for a minute, approximately 96% of these microorganisms were eliminated. In another study, Kustarci et al.(14) evaluated the antimicrobial activity of potassium-titanyl-phosphate (KTP) laser and gaseous ozone (2100 ppm/min) in root canals for 120 s. Additionally, Muller et al. (15) also explored resistance of ozone against bacteria. They applied gaseous ozone for 60 s. However, complete elimination of bacteria could not be achieved in these studies (13-15).
After reviewing the results of the application time in these studies (13-15), the current study investigated the antifungal effect of gaseous ozone obtained from HealOzone (2100 ppm/min) on root canals infected with C. albicans for 300 s. According to the results of the present study, gaseous ozone eliminated most of the C. albicans. The results obtained by the abovementioned studies (13-15) exhibited similar antimicrobial efficacy compared to the results of present study. Besides, although the present study used longer application time, complete disinfection was not achieved. It can be the result of insufficient gaseous ozone penetration into the tubules of root canals. Cardosa et al. (24) evaluated the antimicrobial effect of aqueous ozone (3.3 mg/L, 300 s) against C. albicans and Enterococcus faecalis. A significant reduction was observed in the number of C. albicans and E. faecalis when samples were immediately taken from the root canals. Furthermore, aqueous ozone as an irrigant agent was effective against C. albicans and E. faecalis, but it was not able to neutralize all the microorganisms.
Arita et al. (17) examinated the antimicrobial effect of aqueous ozone (4 mg/L) applied with ultrasonic techniques at different times against C. albicans on acrylic resin plates. Although there was a slight reduction in the number of fungi after 60 s, it took more than 30 min to achieve complete microbial elimination. Nagayoshi et al. (10) studied the antimicrobial activity of aqueous ozone (4 mg/L) on oral microorganisms and dental plaque. As a result, although 2 mg/L of aqueous ozonefor 120 s had a remarkable antimicrobial effect,4 mg/L of aqueous ozone killed the whole C. albicans. In the present study, aqueous ozone with ultrasonic technique indicated stronger antifungal effect than aqueous ozone with manual technique. Additionally, ultrasonication of aqueous ozone was found as effective as NaOCl against C. albicans. The results of previous studies (10, 17, 24) support the results of the present study. Unlike these studies (10, 17, 24) the current study used the root canals of extracted human teeth and the direct counting method of fungi, which was carried out after 24 h of incubation at the microbiological stage.
According to the findings of the current study, gaseous ozone can be used for disinfection of infected root canals, but it is not adequate when used alone for root canal sterilization. Moreover, using aqueous ozone combined with ultrasonic technique is highly effective in terms of disinfection of the root canal and may be used instead of NaOCl in root canals. Further research is needed to investigate the availability of aqueous ozone that has more adequate properties for clinical endodontic treatments.