1. Background
Cutaneous leishmaniasis (CL) is more common compared to the other forms of leishmaniasis. Uncontrolled migrations from rural to urban areas, building constructions, and mass accumulation of garbage, provide suitable environments for sandflies reproduction (1, 2). CL is endemic in many parts of Iran including Mashhad, center of Khorasan-Razavi province (3-5). Torghabeh - Shandiz district is a touristic countryside zone at the west of Mashhad on the mountainside of Beenalood. Because of its natural beauty and bushy gardens, it has always been considered a lovely promenade for nature-lovers. This area is endemic for CL. Formerly, by Immunological and molecular methods such as isoenzyme electrophoresis, ELISA monoclonal antibody and PCR, it was approved that both anthroponotic cutaneous leishmaniasis (ACL) and zoonotic cutaneous leishmaniasis (ZCL) are present in Mashhad, but adequate information about CL in Torghabeh – Shandiz district is not available (1, 5-10).
2. Objectives
The main goal of this study was to complete the leishmaniasis map of Khorasan-Razavi province. In the previous studies, the pattern of CL map has been investigated in several districts of this province e.g. Mashhad, Nishaboor, Sabzevar, Khaf and Taybad (1, 5, 6, 9-13). In the present study, the situation of CL in Torghabeh – Shandiz district has been investigated by molecular methods.
3. Patients and Methods
This study was performed at Mashhad University of Medical Sciences and Jahad Daneshgahi of Mashhad from January to December 2010. Based on the inclusion and exclusion criteria, the study population included 164 people who had at least one skin lesion suspected to CL and referred to the Torghabeh - Shandiz health centers. The number of collected samples in Torghabeh and Shandiz health centers were 65 and 99, respectively. Before sampling, an informed consent form was obtained and a questionnaire was filled out for each individual. We prepared two slide smears from the skin lesion of each patient. Geimsa-stained slides were observed under a light microscope with high magnification (1000X). All positive and negative results for amastigote were recorded. The slides were kept in a box for molecular testing. DNA extraction was performed by scraping the smears and using QIAamp DNA Mini Kit (Qiagene,Germany).
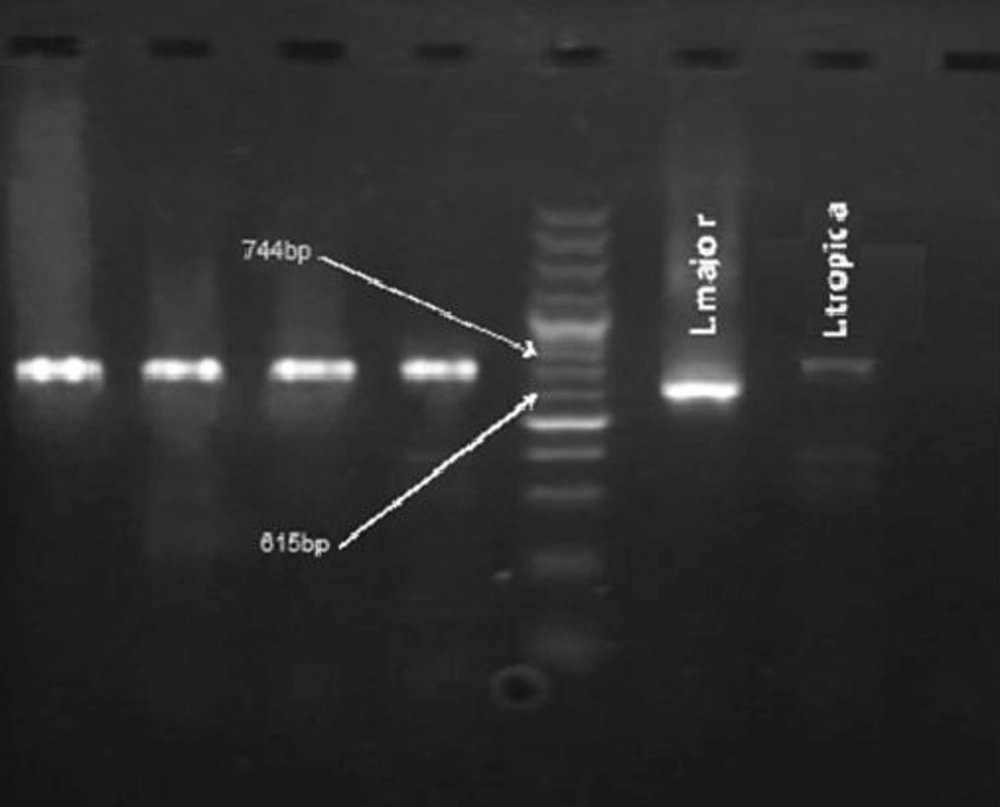
Primers used in this PCR reaction were synthetic oligo-nucleotides with kDNA pattern of Leishmania donovani. The sequences are: F: (5' TCGCAGAACGCCCCTACC' 3') and R: (3' AGGGGTTGGTGTAAAATAGG 5' (Tuba Negin, Iran) (12). DNA replication pattern of Leishmania produced bands for L. major / 615 base pair (bp) and for L.tropica / 744 bp using the abovementioned primers. The test was set up at different annealing temperatures (56, 58, 60 and 62°C) and concentrations of MgCl2 (0.5 to 4 µM). Finally, the sharpest bands were observed at 60°C and 2 µM of MgCl2 for both species. At last, the total volume of reaction was 25 µM of which 5 µM belonged to DNA. In each PCR test, two standard samples of parasites (strain MRHO/IR/75/ER of L. major and strain MHOM/IR/01/yaza of L. tropica) and a negative control sample were used to monitor the reaction. PCR products were analyzed by 1.5% agarose gel electrophoresis and observed by UV Doc system (Doc-008.XD).
4. Results
Direct examination of 164 samples obtained from the skin lesions of the patients showed 122 positive results for Leishmania, but via PCR, Leishmania DNA was isolated from 136 (82.9%) smears. The number of patients from Torghabeh and Shandiz were 57 and 79, respectively. All of the positive samples were identified to be L. tropica (Figure 1).
Among 136 patients approved for CL, 70 (51.5%) were female and 66 (48.5%) were male. Chi-square test indicated a homogenous distribution of infection risk (P = 0.732). The highest rate of CL was observed among the age groups of 1 - 10 and 21 - 30 years respectively. Based on different age groups, Chi-square test showed no homogenous distribution of patients (P < 0.001). Of 136 patients, 59 had only one skin lesion, 22 had two and the rest had three or more. Statistically, no significant difference was observed in both sexes related to the number of skin lesions.
5. Discussion
Cutaneous leishmaniasis (CL) is one of the major health problems and covers a wide area in Iran. Epidemiology of CL has changed in the past two decades in our country. Migration from the neighboring countries such as Afghanistan and Iraq, rural to urban habitations and numerous constructions have provided suitable conditions for the disease transmission (14). Based on the epidemiological studies, Mashhad district has been known as a focus area for ACL for sixty decade (3). Mohajery et al. have reported the existence of ZCL in addition to ACL in this city. Subsequent investigations have confirmed this result (10).
Sabzevar is one of the districts located at the margin of the desert which was previously considered as an endemic focus area for ACL; but a recent study showed the presence of both L. major and L. tropica in this district (12). Since 30 years ago, Torghabeh – Shandiz district with a rural environment has been known as the center of CL, but there were no evidence about Leishmania causative agents. In the present study, we have shown the presence of ACL using molecular techniques in this county for the first time. In this study, of 164 individuals having cutaneous lesions, 136 (82.9%) were positive for ACL, resulted from PCR method. Absence of L. major in these rural areas may be due to the absence of vectors and/or reservoirs of ZCL. The same result was observed in another study in Neishaboor (11).
In this study, DNA extraction of the specimen was performed on the slides, which makes it possible to identify the species in addition to rapid diagnosis of the disease. This method does not require culturing or mass production of promastigotes. Similar result has been obtained by some investigators (15). Moreover, Culture of all positive samples is not always a successful method. .Fata et al. have shown , extraction of Leishmania DNA from samples was preserved on the Whatman paper and direct smears gave a better result in comparison with extraction of DNA from promastigotes obtained from culture (6).
All of the samples were obtained from the positive smears besides 14 (10.3%) negative smear samples were positive for Leishmania using PCR method. It means that among 136 positive samples indicated by PCR, 14 had not been positive by direct smear method, which demonstrates the higher sensitivity of PCR in comparison with direct microscopic examination.These findings have been approved by previous studies in Iran and other countries which implies the fact that PCR on the direct slides for identification of Leishmania species is a rapid method with high sensitivity and specificity (15, 16). In another study which was performed on negative smears obtained from skin CL lesions, the result was significantly similar (17).
The results showed that despite the existence of L. major in the Mashhad district, these touristic countrysides with rural environments have remained as pure focus areas of L. tropica. Furthermore, Giemsa-stained slides are good samples for DNA extraction and there is no need for extra samples which can cause patients' suffering. Moreover, they are easily stored and sent to the molecular laboratories (18). CL is endemic in Torghabeh - Shandiz district. This countryside with a rural texture is an active focus area for ACL. Further studies are recommended to discover the vectors and probable reservoirs in this area. According to this study, PCR is a very rapid and sensitive diagnostic method, hence molecular tests are strongly recommend to be done at least for direct negative slides. It can be effective in acceleration of the healing process.