1. Background
Plants have been a veritable source of medicine. For many centuries people have been trying to relieve and treat diseases using different plant extracts and formulations (1). The interest in plants antimicrobial properties has been revived because of the current problems associated with the use of antibiotics (2). Nowadays, the fact that microorganisms tend to develop drug resistance, besides the side effects of certain antibiotics has offered considerable potentials for the development of new effective antimicrobial and antioxidant agents; medicinal plants are prolific sources.
Antimicrobials of plant origin are effective in the treatment of infectious diseases and reduce many side-effects that are often associated with synthetic ones (3, 4). The increasing prevalence of antibiotic resistance is a major health concern, worldwide. The World Health Organization (WHO) and the European Commission (EC) have recognized the importance of studies on the emergence and determinants of antimicrobial resistance and the need for strategies to control drug resistance (5).
Myrtle (Myrtuscommunis L.) is an evergreen sclerophyll perennial shrub belonging to the Myrtaceae family that is spontaneously growing throughout the Mediterranean area (6). Myrtle is very aromatic because of the high essential oil contents of its leaf, flowers and fruit glands. Myrtle has demonstrated important antimicrobial and antifungal activities to treat bacterial and fungal diseases (7). The essential oils and components extracted from the leaves have both antimicrobial activity and ability to neutralize free radicals and prevent unsaturated fatty acid oxidation (8). Myrtle is traditionally used as an antiseptic, disinfectant drug and hypoglycaemic agent (6, 9).
Dermatophytes are a unique group of fungi that infect keratinous tissues of lower animals and humans (10). They are characterized by their ability to invade the superficial layers of the epidermis, particularly, the stratum corneum and the high keratin-concentration containing appendages, the hair and nails of the living host (11, 12). Only under exceptional circumstances are able they survive or proliferate within the deeper tissues of the body (13). These fungi have a worldwide distribution, and at present, there are 40 recognized species in the dermatophyte genera (12).
About 25 species belonged the genera Epidermophyton, Microsporum and Trichophyton are presently known to infect man (10). It can be caused by keratinophilic and keratinolytic dermatophytes, particularly Microsporum canis, M. gypseum and Trichophyton mentagrophytes (10,14,15,16). During the last decades, many antifungal agents have been developed and have become available for the treatment of dermatophytosis, which are confined to a relatively few number of chemical groups. In addition, the occurrence of drug-resistance or side effects in clinically isolated strains leads to failure in the treatment of mycosis (17). Thus, effective antifungal agents, which are highly effective and safe, are necessary and important for analyzing the antibiotic-susceptible and -resistant strains.
The advent of synthetic antimicrobials in the mid-20th-century leads to lack of interest in plants as a natural source of antimicrobial drugs (1). During the recent years the situation has changed and the field of ethnobotanical research has developed (18). It is necessary to scientifically investigate the plants which have been used in traditional medicine to improve the quality of healthcare. Since now, this is the first report of its kind to show antifungal activity of flavonoids of Myrtle as effective components against M. canis, M. gypseum and T. mentagrophytes.
Bioautography is a laboratory technique to detect substances affecting the growth of organisms in complex mixtures such as plant extracts. Bioautography methods are usually divided into three categories: Agar diffusion or contact bioautography, Immersion or agar-overlay bioautography and direct bioautography. In contact bioautography, antimicrobials diffuse from a TLC plate or paper to an inoculated agar plate. In immersion bioautography, the chromatogram is covered with a molten, seeded agar medium. In direct bioautography, a developed plate is dipped in the suspension of microorganisms growing in a suitable broth or this suspension is sprayed onto the plate. The plate is incubated and microorganisms grow directly on it. Hence, separation, preconditioning, incubation and visualization are performed directly on the plate. For location and visualization of antibacterials, Tetrazolium salts are usually used, which are converted by the dehydrogenases of living microorganisms to intensely colored, formazan. Since, the bacteria are killed by antimicrobials on the TLC plate the color is not produced in places of antibacterial spots so-called zones of inhibition that are pale on a colored background are formed (19).
2. Objectives
The primary objective of the present study was developing a new method for evaluation of antifungal activity of hydroalcoholic extract of Myrtle and its fractions on dermatophytes by disk diffusion, tube dilution and bioautography.
3. Material and Methods
3.1. Plant Material
The leaves of M. communis were collected from Charfarsakh of Shahdad, Kerman, Iran. Then were dried and powdered. A voucher specimen No. 1357 - 1 has been kept in Herbarium of Faculty of Pharmacy, Kerman University of Medical Sciences, Kerman, Iran.
3.2. Microorganisms and Growth Conditions
The species used for this study were: M. canis ATCC 32903, M. gypseum ATCC 14683 and T. mentagrophytes ATCC 1481 (var. interdigitale) which were kindly provided by Tehran University of Medical Sciences. The fungi were kept on Sabouraud dextrose agar (SDA, Merck, Germany) slants at 4°C and monthly sub-cultured throughout this study.
3.3. Preparation of Extracts
All solvents used in the experiments (petroleum ether, dichloromethane, ethyl acetate and methanol) were purchased from Merck (Germany). The air-dried and powdered leaves of plant (20 g) were soaked with 80% methanol (150 mL). The total methanolic extract of the plant leaves was prepared by maceration in triplicate for 48 hour, evaporated under vacuum at 50 °C, lyophilized and kept at −10 °C. The antimicrobial effects of the extracts were measured against dermatophytes.
3.4. Preparation of Fractions
Various fractions were prepared from hydroalcoholic extracts based on their polarity. 10 mL of methanol was added to dried hydroalcoholic extracts (1 g) then it was suspended in 15 mL of water and repeatedly extracted with different solvents. The resulting extracts were evaporated under vacuum to provide the following fractions: petroleum ether, dichloromethane, ethyl acetate and the remaining aqueous solution (which will be called as the hydroalcoholic fraction. Fractions with different polarities were created using successive extraction and solvents with different polarities with an increasing manner.
3.5. Evaluation of Antifungal Activity
3.5.1. Paper Disk Diffusion Assay
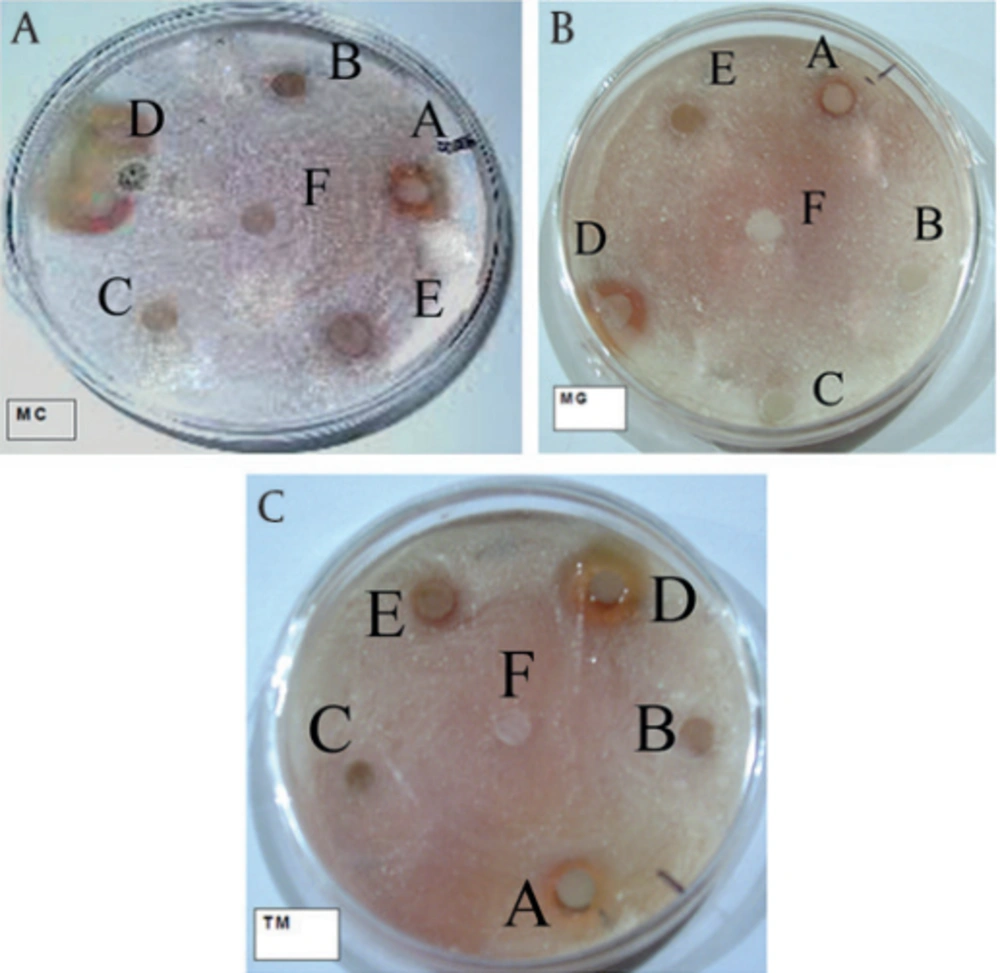
Test plates (diameter, 8 cm) were prepared with SDA and inoculated (100 μL) with a spore suspension in sterile dissolution of 0.9% saline. The concentration of suspension was adjusted to 105 CFU/mL. 6-mm diameter sterile filter paper discs (Hi-Media) were soaked in 10 μL of methanolic and other various extracts solution (10 mg/mL) and air dried (100 µg/disk); then the discs were placed on the surface of the plates. Griseofulvin disk (6 µg/disk) was used as positive and methanol as negative control. Aqueous tetrazolium salt solution (INH from Merck, 2 mg/mL) also was used as an indicator. The plates were incubated at 28ºC for 48 hours. The experiment was performed in triplicates to minimize the error rate. After incubation, the antifungal activity was evaluated by measuring the inhibition zones. The extract with maximum inhibitory effect was selected for bioautography (20).
3.5.2. MIC Determination Based on Tube Dilution Method
Serial dilutions of various Myrtle leaf extracts stock solutions (10 mg/mL) were incorporated into 0.5 mL of SDA in glass tubes in order to adjust to the concentrations of 5 – 0.187 mg/mL. All tubes were inoculated with 50 µL standardized inoculums (105 CFU/mL) of each three organisms and incubated at 26◦C. The last tube of SDA containing 50µL inoculums served as positive control. The SDA served as negative control. The whole set up in duplicate was incubated at 25 – 30◦C for 8 days. Minimum inhibitory concentration (MIC) was defined as the lowest concentration of the extract with no growth after incubation (20).
3.5.3. Bioautography Method
3.5.3.1. TLC
A thin layer chromatography (TLC) was developed for carrying out bioautography. The same solvent systems were used for TLC and bioautography. The following solvent systems were used: ECM: Ethyl acetate, Chloroform, Methanol (35: 55: 10) and (32: 53: 15); ECA: Ethyl acetate, Chloroform, Acetic acid (45: 50: 10); ETMW: Ethyl acetate, Trifluoroacetic acid, Methanol, Water (10: 1: 1: 1) and (10: 0.5: 0.1: 0.1) and (10: 0.1: 0.05: 0.05); EFGW: Ethyl acetate, Formic acid, Glacial acetic acid, Water (100: 11: 11: 27); EC: Ethyl acetate, Chloroform (60: 40); BAW: Butanol, Acetic acid, Water (4: 1: 5 upper phase); CAF: Chloroform, Acetone, Formic acid (75: 16.5: 8.5) The best solvent system was applied for bioautography was ETMW (10: 0.1: 0.05: 0.05). The TLC plates were read at 254 nm and 366 nm using UV chamber.
Immersion bioautography: 200 μL of methanolic extract of ethyl acetate fraction (10mg/mL) were applied to pre-coated Silica gel GF254 2 × 7-cm TLC plate (Merck), developed with ETMW (10: 0.1: 0.05: 0.05 v/v) and dried for complete removal of solvents and overlaid by agar seeded with an overnight culture of dermatophytes (4 mL of inoculums (105 CFU/mL) and 16 mL of SDA). The plate was incubated at 25 – 30◦C for 8 days. Then were sprayed with an aqueous solution of tetrazolium salt (2% INH (Merck, Germany) in water). The areas of inhibition were pale on a purple colored background (19).
The Rfvalues were compared with the Rf value of the active spot with antifungal activity on the bioautograms.
3.6. Statistical Methods
Antifungal disk diffusion and MIC determination based on tube dilution method were performed in triplicate. Inhibition zones diameters (mm) of various Myrtle leaf extracts were measured and expressed as mean ± SD.
4. Results
4.1. Dried Weight of Extracts
The weight of different Myrtle leaves extracts was as follow : Total methanolic extract; 45% w/w, Petroleum ether fraction; 10%, Dichloromethane fraction; 4%, Ethyl acetate fraction; 6%, and Hydroalcoholic fraction; 25% w/w.
4.2. Evaluation of Antifungal Activity
4.2.1. Paper Disk Diffusion Assay
Each test was repeated three times for each fungus. The zones of inhibition showed in Figure 1. Ethyl acetate and pure methanolic extractions respectively had the highest antifungal effects against three fungal strains. M. canis after seven days while M. gypseum and T. mentagrophytes after three days showed the inhibition zones (Table 1).
aDisks had 6 mm diameter, n = 3
Abbreviations: TA: Total methanolic extracts, PE: Petroleum ether fraction, DM: Dichloromethane fraction, EA: Ethyl acetate fraction, HA: Hydroalcoholic fraction
4.2.2. MIC Using Tube Dilution Method
Ethyl acetate and total methanolic extracts had the lowest MIC against three fungal strains (Table 2).
Abbreviations: TA :Total methanolic extract, PE: Petroleum ether fraction, DM: Dichloromethane fraction, EA: Ethyl acetate fraction, HA: Hydroalcoholic fraction, MIC: Minimum Inhibitory Concentration
4.2.3. Bioautography
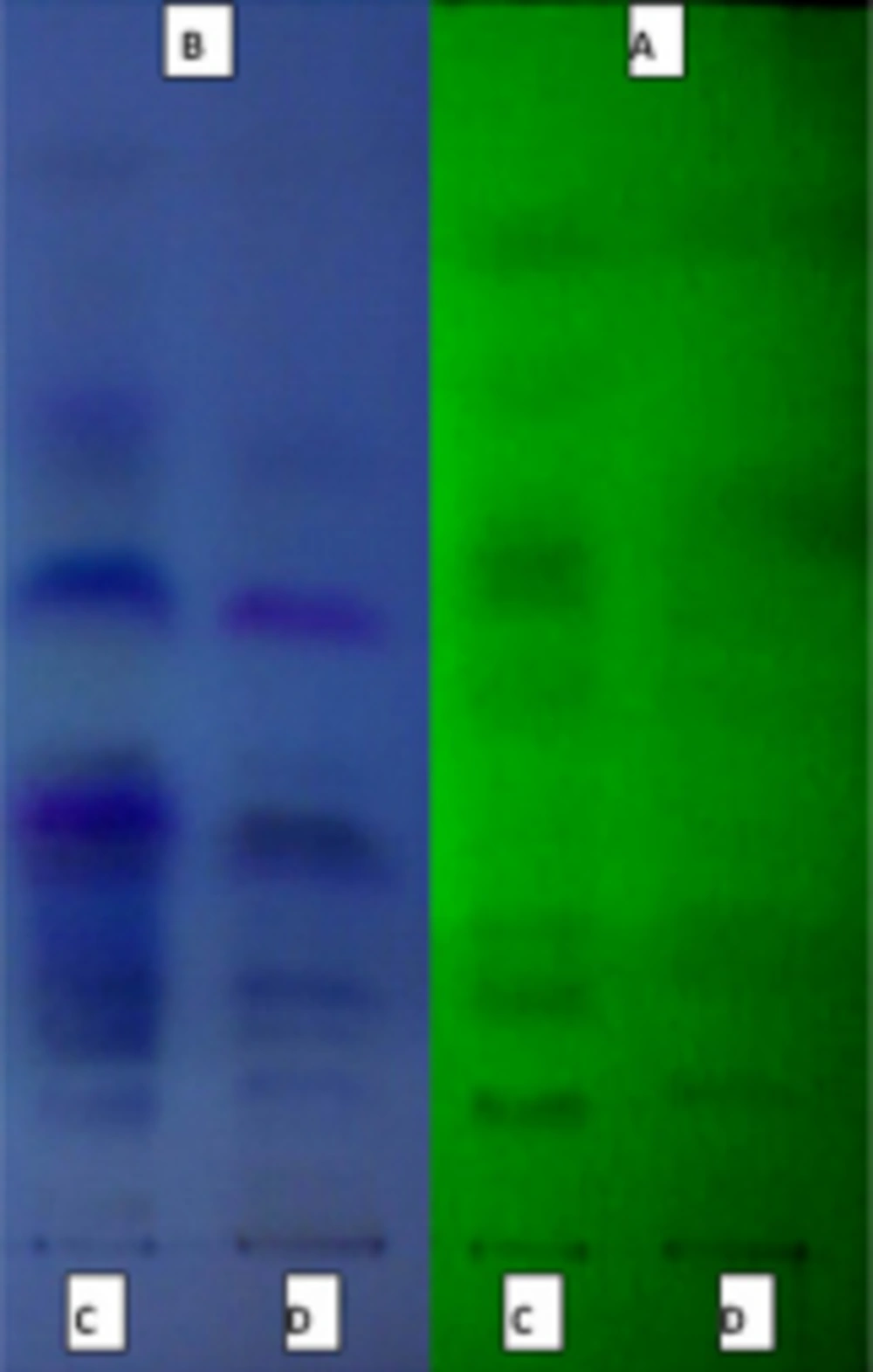
In this method, the ethyl acetate and total methanolic extract that had large inhibitory zone, were separated by solvent system (ethyl acetate, trifluroacetic acid, methanol, water (10: 0.1: 0.05: 0.05)) on TLC (Figure 2).
Because of the ethyl acetate fraction of hydroalcoholic extraction showed the highest activity, we used this fraction for this bioautography assays. The best antifungal effects of all three fungi, was measured in R f : 0 - 0.3 (Figure 3) with 4 spots in 365 nm and 2 spots in 254 nm.
5. Discussion
The present study was designated to determine the antifungal activities of various fractions of Myrtle leaves methanolic extracts. The results of this research were confirmed by disc fusion method and bioautography. These activities are contributed by effective compounds of the present fractions. A TLC method was developed to carry out the bioautography. The active compound may be flavonoid. Existence of flavonoids in tested fractions could be the important medicinal properties of M. communis leaves. The previous studies showed that phenolic compounds such as flavonoids, phenolic acids and tannins are widely distributed in plants (21), which have attracted much attention, due to their antioxidant activities and free radical-scavenging abilities, which potentially have beneficial implications for human health (22).
Romani et al. (23) concerning the phenol composition of myrtle leaves. They reported that the most abundant polyphenolic class in myrtle leaves was hydrolysable tannin that received an increased attention because of some new findings related to their biological activities such as anti-cancer, antiviral and inhibition of lipid peroxidation (24). Yoshimura et al. (25) isolated and identified ten phenolic compounds from myrtle leaves including four hydrolysable tannins (oenothein B, eugeniflorin D2, tellimagrandins I and tellimagrandins II), polyphenolic compounds (gallic acid and quinic acid 3,5-di-O-gallate), and myricetin glycosides (myricetin 3-O-b-D-xyloside, myricetin 3-O-b-D galactoside, myricetin 3-O-b- D-galactoside 6-O-gallate and myricetin 3-O-a-L-rhamnoside) (7). All these studies had led to investigate the phenol composition of myrtle leaf but no data was available regarding the phenol composition of myrtle stem and flower. Also Koroishi et al. (26) showed that the 90% hydroalcoholic extract of Piper regnellii leaves had a strong activity against the dermatophyte fungi T. mentagrophytes, T. rubrum, M.canis and M.gypseum.
Gardeli et al. (27) had only studied the total phenol content of the methanolic leaf extract of Greece myrtle which was tenfold higher (373 mg GAE/g) than that found in this study, and Hayder et al. (28) studied the effect of extraction solvent on antiradical activity of myrtle leaf extracts from Tunisia and they mentioned that polar extracts such as aqueous extract (IC 50 = 1.90 mg/mL) and methanol extract (IC 50 = 6.50 mg/mL) showed higher antiradical scavenging activity than polar extracts (hexane) and essential oil which showed a high IC 50 at 100 mg/mL.
In the present work, comparing the activity of the active fractions of total methanolic crude extract showed the same results against all three fungi. Possible synergy would explain many failed attempts to isolate single, active compounds from medicinal plants. It is hoped that this study will enthused investigations at the molecular level of possible medicinal plant synergisms. Although the present study, investigated the In vitro antidermatophyte activity, the results prove the ethno-botanical use of the Myrtle extract for the treatment of various fungal related diseases. However, In vitro data may be helpful to determine the potential usefulness of the plants for treatment of dermatophyte infections (26). In terms of conservation, the results showed that leaves components are useful for antifungal uses. We suggest more conclusive studies to evaluate these biological components.