1. Background
Streptomyces avermitilis is an aerobic, Gram positive and mesophilic Actinomycete, forming extensively branched substrate mycelium and aerial hyphae. The hyphae are differentiated into long, compact spiral chains which become more open as the culture ages, and are specialized for the production of the avermectin complex (1). Avermectins are secondary metabolites having anthelmintic and insecticidal properties and have extensively been used in agricultural and animal health cases. They have been produced from S. avermitilis by fermentation (1). Strain improvement strategies and better production conditions are very important to enhance the yield of secondary metabolites in any fermentation process (2). The concentration on secondary metabolites produced from wild strains is very low due to the complicated economical procedure (3).
Hundred times greater yields of metabolites can be achieved through suitable strain improvement techniques (4). Mutagenesis is the most reliable and widely-used tool for strain improvement (5). Induced mutations using UV rays, X-rays, γ-rays, laser, neutron, and chemophoresis are in practice for organism breeding (6). Methyl methane sulfonate (MMS), hydroxyl amine (HA), and N-methyl-N-nitro-N-nitrosoguanidine (MNNG) are the physical methods to induce mutations (7). MNNG is highly specific in producing GC-AT transition mutations and this limits its usefulness as a mutagen (8).
Chemical modification of nucleotides is supposed to be induced using alkylating agents such as Ethylmethane Sulfonate (EMS). It results in mispairing and base changes in the nucleotide sequence (9). EMS mutagenesis is useful for producing breeding lines (10). EMS produces C-T changes resulting in C/G and T/A substitutions (11). 7-ethylguanidine hydrolysis results in G/C to C/G or G/C to T/A transversions while 3-ethyl adenine pairing errors cause A/T to G/C transitions (12). Treatment with ethidium bromide (EB) usually results in bald mutants leading to no sporulation (13).
The most important and convenient physical method to obtain broad spectrum mutations is UV radiation. It is safe to use UV light as a mutagen, compared to chemical mutagenesis (14). Improved secondary metabolite production from industrial microbe strains has been obtained by random mutagenesis and fermentation screening (15). Most of the Streptomyces members are genetically unstable, and morphologically stable mutants are needed for strain improvement strategies (16, 17).
2. Objectives
The present study was conducted for production and screening of avermectin B1b (Figure 1) hyper-producing mutant strain of S. avermitilis 41445 by means of physical (UV radiation) and chemical mutagenesis (ethyl methane sulfonate and ethidium bromide). The main objective of the study was to enhance the production of avermectin B1b through mutagenesis.
3. Materials and Methods
3.1. Microorganism and Maintenance of Culture
S. avermitilis DSM 41445 provided by Deutsche Sammlung von Mikroorganismen and Zellkulturen (DSMZ) GmbH was used throughout the study. S. avermitilis DSM 41445 was maintained on medium 65 as specified by DSMZ. The medium 65 (Yeast extract malt extract glucose medium) (Merck, Germany) consisted of glucose 4.0 g, yeast extract 4.0 g, malt extract 10.0 g, and CaCO3 2.0 g (g/L in distilled water) (18). The medium was adjusted to pH 6.5 before sterilization. After sterilizing at 121°C for 15 minutes, the medium pH was adjusted at 7.0 by adding CaCO3 (20). The medium was then inoculated with S. avermitilis 41445 and incubated at 28oC in a water bath shaker at 150 X g until it was converted to a brownish liquid. Nutrient agar (Merck, Germany) slants were used for the culture to streak, followed by incubation at 28oC for 24 hours to be stored. All the mutant microbial cultures were maintained on nutrient agar slants (2.8%w/v 2.8 g nutrient agar dissolved in 100ml of distilled water). The incubation temperature for the culture growth was 28°C, because the S. avermitilis culture grows well at 28°C and 37°C (2).
3.2. Experimental Protocol for Microbial Mutation
3.2.1. UV Mutagenesis
The S.avermitilis DSM 41445 spores were exposed to UV rays at a distance of 10 cm from the UV lamp (DESAGA, Sarstedt-Gruppe, MinUVIS UV Lamp) (λ = 320nm) for 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60 minutes. All these exposures were performed in a dark room to avoid any photoreaction in the production of mutants. The spores were then spread in plates containing nutrient agar. The plates were incubated at 28oC for 24 hours The method employed for UV mutagenesis was described earlier by Khattab in 2012 (19) and used in the present study with little modifications. The lethality rate, mutation rate, and positive mutation rate were calculated using the below equations (20):
Lethality rate = No. of colonies after UV treatment / No. of colonies without UV treatment (T/U) × 100%
Mutation rate = total colony formation unit of the mutant strain / No. of colonies after UV treatment (M/T) × 100%
Positive mutation rate = CFU of the mutants with avermectin B1b production more than that of the parent S.avermitilis DSM 41445 / total colony formation unit of the mutant strain (P/M) × 100%
Here, U = No. of colonies without UV treatment
T = No. of colonies after UV treatment
M = total colony formation unit of the mutant strain
P = CFU of the mutants with avermectin B1b production more than that of the parent S.avermitilis DSM 41445.
The plates with highest lethality rate obtained after UV exposure were selected and all the colonies from those plates were incubated in nutrient agar slants for 24 hours at 28°C. The selected mutant colonies were further screened through avermectin production ability to determine the positive mutation rate (20).
3.2.2. Chemical Mutagenesis
For chemical mutagenesis, a 12-hour-old culture of S.avermitilis 41445 in nutrient broth was used to make the serial dilutions up to 10-7 in sterilized normal saline. Various concentrations (10 µL/mL, 20 µL/mL, 30 µL/mL, and 40 µL/mL) of EB were used for the mutation of S.avermitilis 41445 per mL of 10-7 dilution. Effect of each concentration was studied at different time intervals (10, 20, 30, 40, 50 and 60 minutes).
Similarly in case of EMS, 1 µL of EMS was used for 1 mL of dilution. Then, 0.3 mL of the above dilution was taken and poured in the Petri plates each of which containing nutrient agar. The plates were incubated at 28°C for 24 hours. Thereafter, the percentage of survival rate in each plate was calculated using the equation mentioned below, and mutants with the lowest survival rates were selected from the plates (21).
S = (Ni - Nd /Ni) × 100
Where, S = survival rate
Ni = Initial CFU
Nd = Colony formation unit (CFU) after mutation
3.2.3. Seed Medium
A loopful of the 24-hour-old culture of S.avermitilis 41445 and all the selected produced mutants were transferred discretely into sterilized seed media. All seed media were placed at 31°C in a water bath shaker (Eyela, Japan) for 24 hours at 150 X g. The seed medium with pH 7.0 ± 2 contained glucose 4.0 g, yeast extract 4.0 g, malt extract 10.0 g, and CaCO3 2.0 g (g/L in distilled water) (22).
3.3. Production of Avermectin B1b
Production of avermectin B1b from S.avermitilis 41445 and all the produced selected mutants were studied independently in growth medium named Synthetic medium 2 (SM2). Each production medium was inoculated with 5 mL (10% v/v) of inoculum medium separately. After transferring the seed medium, each growth medium was incubated at 31°C in water bath shaker for 10 days at 150 X g. Composition of the growth medium was soluble corn starch 50.0 g, KCl 0.1 g, NaCl 0.5 g, Yeast extract 2.0 g, MgSO47H2O 0.1 g, CaCO3 0.8 g and α-amylase 0.1 g (g/L). pH of the medium was adjusted at 7.2 ± 0.2. All the experiments were performed in shake flasks containing 50 mL of fermentation medium separately, using the method described earlier (23).
3.4. Extraction of Avermectin B1b
The fermentation broth from each fermentation flask was centrifuged at 4°C for 20 minutes at 8000 X g (H-1500FR, Japan) for the extraction of avermectin. Avermectin being an intracellular molecule has to be extracted from the cell biomass, and for this purpose, cell biomass was taken and the supernatant was discarded. The cell biomass in the form of pellet was mixed with an appropriate amount of methanol in a pestle and motor and was crushed hardly to be completely dissolved. The mixture was then centrifuged for separation of the cell biomass and the supernatant was collected for the analysis of avermectin by HPLC.
3.5. HPLC Analysis of Avermectin B1b
For quantitative determination of avermectins produced from S.avermitilis 41445 and all the mutant strains, reverse phase HPLC was employed. About 20 µL of each extracted sample was applied into HPLC (LC-2080 Shimadzu) where C18 column (SMA C-18) and detector (UV variable wavelength detector STD-M20A Shimadzu) were used for separation of components, and individual components were eluted by methanol: acetonitrile (98 : 2 v/v) at a flow rate of 0.5 mL/min with a UV absorbance at 246 nm (24).
4. Results
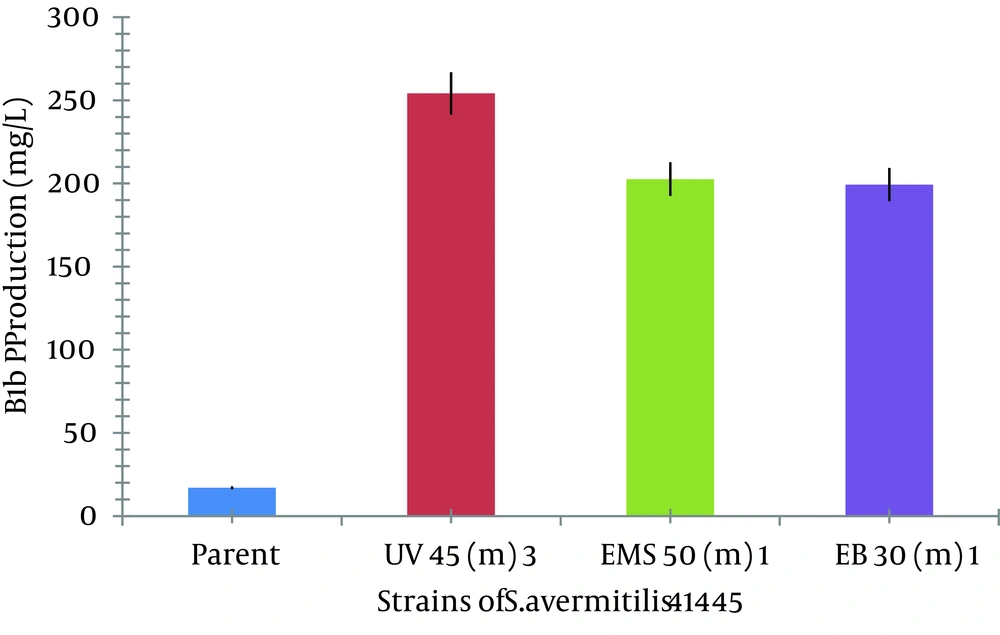
UV exposure for 45, 55, and 60 minutes resulted in 20%, 16.66%, and 13.33% survival rates, respectively, as shown in Figure 2. The lethality rate increased from 0.05 to 0.075 with exposure time elevation from 45 to 60 minutes. In our findings, death rate of 80 - 88% was noted in the case of UV radiation. Number of mutant colonies decreased with increase of exposure time. Maximum increase in avermectin B1b production was observed in mutants obtained at exposure time of 45 and 55 minutes. From the HPLC results, it was found that the avermectin B1b-hyper-producing mutant strain obtained from UV radiation was UV 45 (3), which resulted in 14.9496 ± 0.2 folds increase (254.1443 mg/L) in the B1b component as compared to that of the S. avermitilis 41445 (17 mg/L) after 10 days of fermentation, as shown in Figure 3 C. Based on the avermectin B1b production, the estimated mutation rate (RM) was 92.22% and the positive mutation rate (RP) was 8.42% in the present study.
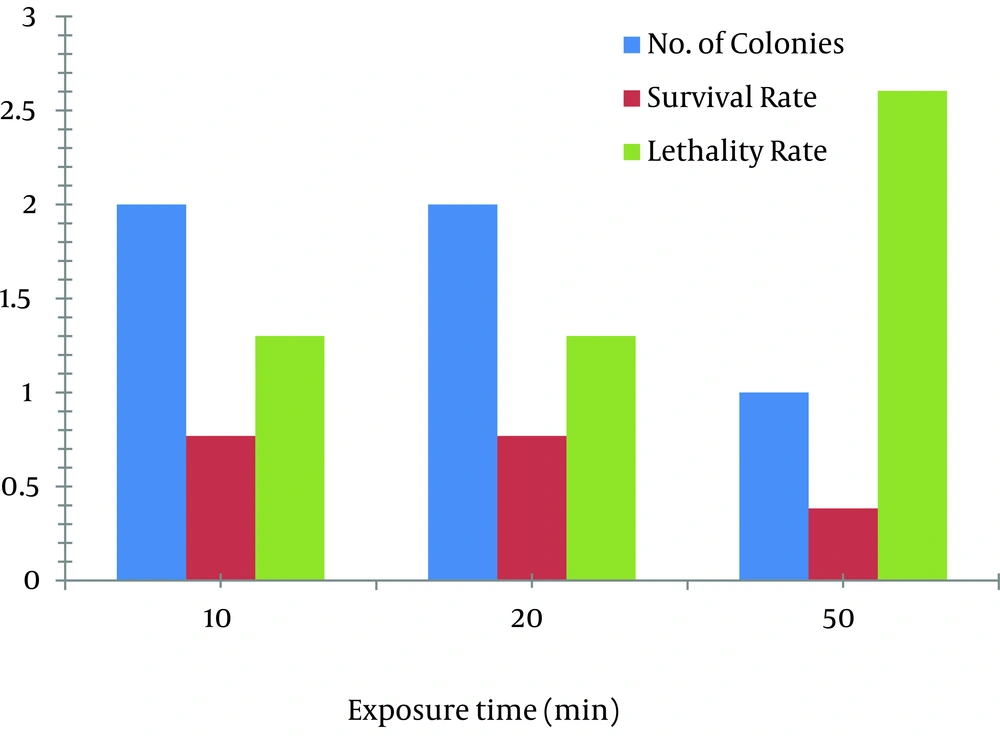
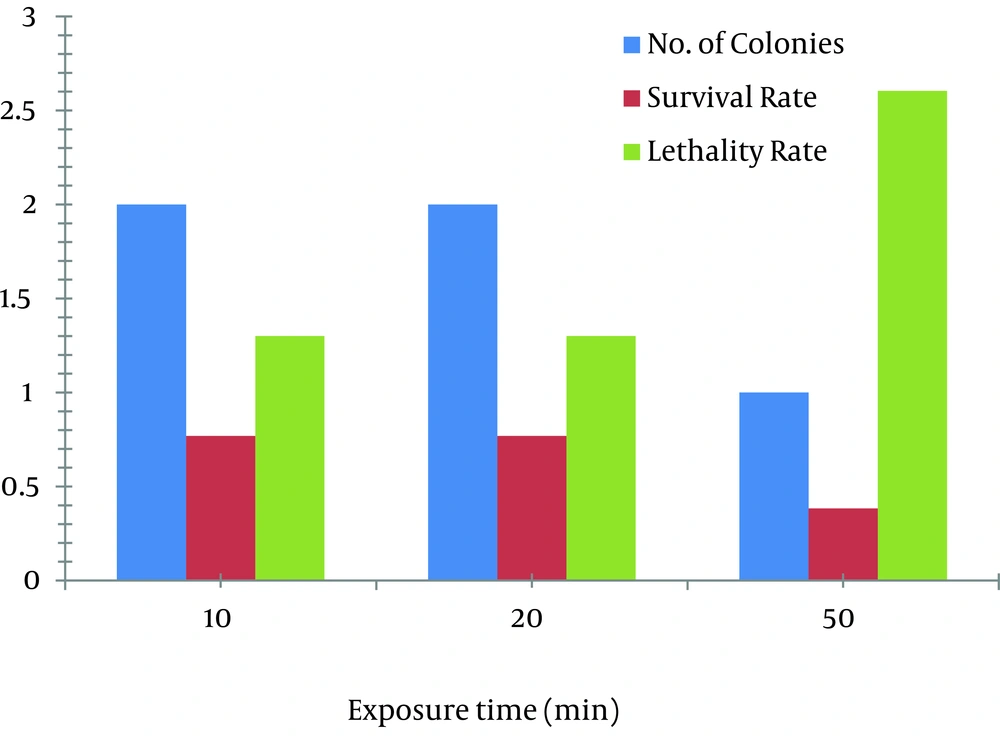
In case of EMS, 1 µL/mL concentration of EMS was used for mutation. At the exposure times of 10, 30, and 50 minutes, the survival rate was decreased from 0.769% to 0.384% and the lethality rate was increased from 1.300% to 2.604%. The mutant obtained at exposure time of 50 minutes, yielded 11 times more production of avermectin B1b (202.63 mg/L) than the parent strain (17 mg/L) as shown in Figure 3 D.
Two mutants obtained at the exposure time of 20 minutes yielded 10.5 times more avermectin B1b production (179.93 mg/L and 192.06 mg/L) as compared to the wild strain of S.avermitilis 41445. Production of B1b component of avermectin by the two mutants obtained at the exposure time of 10 minutes was 90.01 mg/L. Effects of EMS on the survival and lethality rates at various times are shown in Figure 4.
5. Discussion
The strain improvement strategies especially mutagenesis and screening of hyper-producing mutants are very important in the production of secondary metabolites during the fermentation process (25). The genome sequences of S.avermitilis species have been known, but the mechanisms of genes involved in the avermectin production are still not clearly understood (26). UV radiation and chemical mutagenesis have been employed to obtain the avermectin hyper-producing mutants (27).
In the present study, physical and chemical mutageneses have been employed to mutate S.avermitilis 41445 to obtain the avermectin B1b hyper-producing mutant. Physical mutagenesis was done using UV radiation. In a previous study, it was reported that to have powerful mutations and effective screening of mutants, lethality rate should be very high (20).
It is reported that the death rate of 70 - 95% has been optimized for enhanced secondary metabolite production (28). Kelner reported an exponential survival curve with variable UV exposures to spores of S. flaneolus except at the beginning, where the killing rate was less than the later portions of the curve. In their study, mutation frequency arose steadily with exposure time, with no evidence of failure rate of increase at high doses (29). It is reported that a decrease in the survival rate of S. venezuelae from 100% to 8% occurred as a result of UV mutagenesis when the time of UV exposure was increased from 0 to25 minutes (21). In another study, survival rates of 8% and 5% were observed at UV exposure of 100 and 120 seconds, respectively, showing a gradual reduction in the survival rate with increasing UV exposure on S. fradiae NRRL-2702. The mutants at these exposure times resulted in enhanced tylosin production, compared to the wild type strains (30).
It is reported that genes, expression of which are globally regulated, are required for the production of avermectin (31). Results of the present study indicated that the enhanced production of avermectin B1b might be due to some changes in the genetic code of S.avermitilis 41445 as a result of UV radiation. It is reported that chromosomes of S.avermitilis become genetically unstable with UV radiation; thus, mutations in chromosomes occur when UV light falls on the spores (24).
Production of avermectin is directly related to the large number of genes. Over-mutagenesis should therefore be avoided while screening for hyper-producing mutant strain. Optimum dose of mutagen is required in order to get positive mutation. According to the Poisson model of mutagenesis, 37% of the survival rate would be unaffected for enhanced metabolic production (7). Chemical mutagenesis is time and concentration dependent. In the present study, spores of S.avermitilis 41445 were treated with four different concentrations i.e. 10, 20, 30 and 40 µL/mL of EB for time intervals ranging from 10 to 60 minutes.
Avermectin B1b-hyper-producing mutant was obtained from the spores treated with 30 µL/mL concentration of EB in 30 minutes of exposure (199 mg/L of B1b) as shown in Figures 3 A, 3 B3 A. At lower concentrations of EB (10 and 20 µL/mL), the obtained mutants showed almost similar activities (17 mg/L B1b) as was shown by parent strains. In a study conducted by Naveena et al. it was mentioned that by increasing the concentration of chemical mutagens, the survival capacity of the mutants get adversely affected (21). In the present study, it is also observed that at a higher EB concentration (40 µL/mL), obtained mutants did not show any production and it resulted in the loss of activity. EB exposure at 50 minutes with 30 µL/mL concentration resulted in the survival rate of 1.33 % with lethality rate of 0.751% shown in Table 1.
| Conc. of EB | Exposure Time | Colonies, No. | Survival Rate, % | Lethality Rate |
|---|---|---|---|---|
| 10 | ||||
| 0 | 150 | 100 | 0.01 | |
| 10 | 140 | 93.33 | 0.010 | |
| 20 | 140 | 93.33 | 0.010 | |
| 30 | 130 | 86.66 | 0.011 | |
| 40 | 120 | 80 | 0.012 | |
| 50 | 120 | 80 | 0.012 | |
| 60 | 100 | 66.66 | 0.015 | |
| 20 | ||||
| 0 | 150 | 100 | 0.01 | |
| 10 | 100 | 66.66 | 0.015 | |
| 20 | 90 | 60 | 0.016 | |
| 30 | 90 | 60 | 0.016 | |
| 40 | 80 | 53.33 | 0.018 | |
| 50 | 80 | 53.33 | 0.018 | |
| 60 | 70 | 46.66 | 0.021 | |
| 30 | ||||
| 0 | 150 | 100 | 0.01 | |
| 10 | 5 | 3.33 | 0.300 | |
| 20 | 10 | 6.66 | 0.150 | |
| 30 | 4 | 2.66 | 0.375 | |
| 40 | 10 | 6.66 | 0.150 | |
| 50 | 2 | 1.33 | 0.751 | |
| 60 | 8 | 5.33 | 0.187 | |
| 40 | ||||
| 0 | 150 | 100 | 0.01 | |
| 10 | 10 | 6.66 | 0.150 | |
| 20 | 10 | 6.66 | 0.150 | |
| 30 | 8 | 5.33 | 0.187 | |
| 40 | 8 | 5.33 | 0.187 | |
| 50 | 6 | 4.00 | 0.25 | |
| 60 | 6 | 4.00 | 0.25 |
In the previous study, it was also reported that treatment of Streptomyces with EB resulted in the bald mutants, and the mutants were not able to produce the earthy odors (32). This reveals that production of secondary metabolites and the structural differentiation are closely linked.
In an earlier research it was testified that prolonged incubation of EMS resulted in DNA damage, causing cells death. A suitable selection of exposure times is therefore mandatory to accomplish a good and fruitful chemical mutagenesis (21). In one of the studies conducted previously, MMS was used as a mutagen for the enhanced production of avermectin from S.avermitilis. This mutagen produces a mutant with 4 times more AVM B1 production than the parent strain (1). EMS Mutations followed the error-prone pathway and directly affected the mispairing of the alkylating bases (33).
Mutants obtained from different sources usually present variations in the avermectin production (33). Our results also showed that mutants produced using different mutagens had different B1b production rates. In each case, the production was enhanced when compared with the B1b production from the parent strain, S.avermitilis 41445, as shown in Figure 5.
| Serial No. | Type of Mutagen | Exposure Time, mmin | Colonies, No. | Number of Colonies Producing avermectin B1b | Concentration of B1b Produced, mg/L |
|---|---|---|---|---|---|
| 1 | UV | ||||
| 45 | 6 | 2 | UV (45) 1=43.51; UV (45) 3=254.14 | ||
| 55 | 5 | 3 | UV (55) 1=72.43; UV (55) 2=200.27; UV (55) 3=64.11 | ||
| 60 | 4 | 4 | UV (60) 1=64.70; UV (60) 2=31.46; UV (60) 3=49.34; UV (60) 4=106.35 | ||
| 2 | EMS | ||||
| 20 | 2 | 2 | EMS (10) 1 = 90.01; EMS (10) 2 = 90.01 | ||
| 20 | 2 | 2 | EMS (20)1 = 179.93; EMS (20)2 = 192.06 | ||
| 50 | 1 | 1 | EMS (50)1 = 202.63 | ||
| 3 | EB | ||||
| 10 | 5 | 2 | EB (10)1 = 70.68; EB (10)2 = 119.48 | ||
| 30 | 4 | 3 | EB (30)1 = 138.43; EB (30)2 = 199.30; EB (30)3 = 63.17 | ||
| 50 | 2 | 2 | EB (50)1 = 52.03; EB (50)2 = 84.96 |
The mutant strain of S.avermitilis 41445, numbered 3, obtained by UV radiation after 45 minutes of exposure, showed the maximum avermectin B1b production and was selected as the avermectin B1b-hyper-producing strain. The mutant was named as S.avermitilis 41445 UV 45 (m) 3 and will be used in the further studies.
In a study conducted earlier, high avermectin producers and avermectin aglycon mutants were obtained from S.avermitilis using NTG and UV radiations as mutagens (35). In the present study, the genetic stability of avermectin B1b-hyper-producing mutant of S.avermitilis 41445 UV 45 (m) 3 was observed by multiple streaking of the mutant on nutrient agar slants and then analyzing the production of desired avermectin B1b component by HPLC, as shown in Table 3. For isolation of the stable mutants, a strategy has been developed to select a NTG-produced mutant for hyper production of spiramycin (36). About 56-time-enhanced avermectin production has been obtained from S.avermitilis ATCC 31267 with mutagenesis (2).
| Generation | Avermectin B1b Production, mg/L |
|---|---|
| 1 | 254.14 |
| 3 | 260.18 |
| 5 | 230.56 |
| 7 | 245.36 |
| 9 | 251.67 |
| 11 | 258.61 |
| 13 | 235.81 |
| 15 | 240.98 |
The criterion of random selection of mutant was adopted for the screening of mutants. EB concentration of 30 µL/mL with the exposure time of 30 minutes was found to be the most effective for yielding mutants of S.avermitilis 41445 with enhanced avermectin B1b production. For EMS mutagenesis, a concentration of 1 µL/ML and exposure time of 50 minutes was found to be appropriate for enhanced production of avermectin B1b. In case of physical mutagenesis by UV radiation, the suitable exposure time to produce the B1b-hyper-producing mutant of S.avermitilis 41445 was 45 minutes. Both physical and chemical mutageneses proved to be efficient in producing hyper-producing mutant strains; however, the best mutagen found in the present study was UV radiation, yielding 14 times more avermectin B1b than the parent strain (17 mg/L).
Therefore, the mutant produced by UV radiation at exposure time of 45 minutes was selected and will be used for further studies.