1. Introduction
Toxoplasma gondii is known as one of the most common infectious protozoan parasites that has a worldwide distribution (1-3). Cats are recognized as the only definitive host of T. gondii, but humans and other warmed- blooded animals and birds are as intermediate hosts. Humans can be infected by ingestion of raw or uncooked meat containing tissue cysts, unwashed vegetables, contaminated water or soil. Congenital toxoplasmosis occurs via the trans-placental route (4). Toxoplasma infection is largely asymptomatic, but in those individuals who are immune-compromised with AIDS, malignant patients under chemotherapy or organ transplant recipients can become disseminated and cause severe toxoplasmosis and/or encephalitis (5-7). In the present article two cases of cerebral toxoplasmosis in leukemia patients from Ahvaz, southwest Iran is reported.
2. Case Report
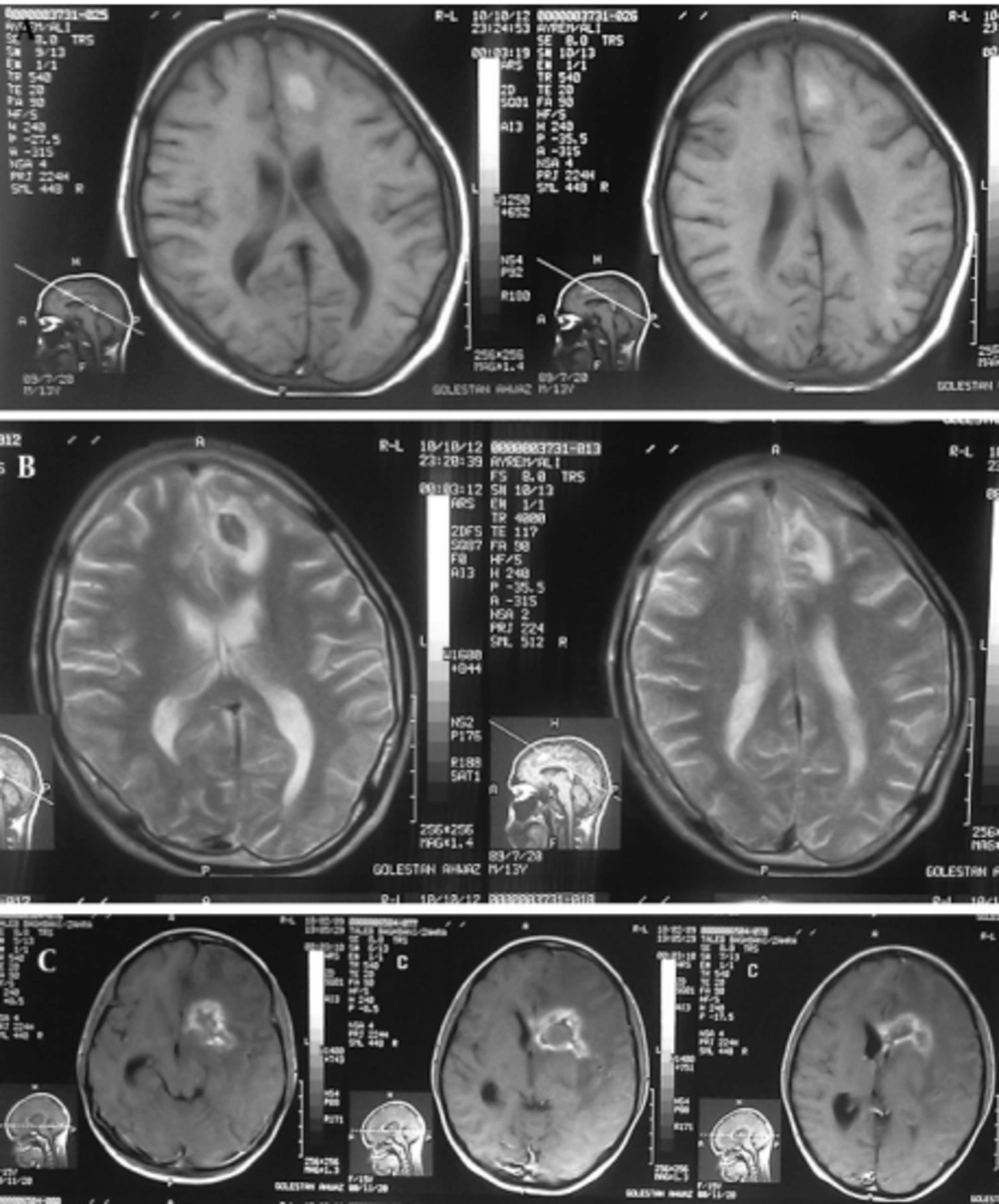
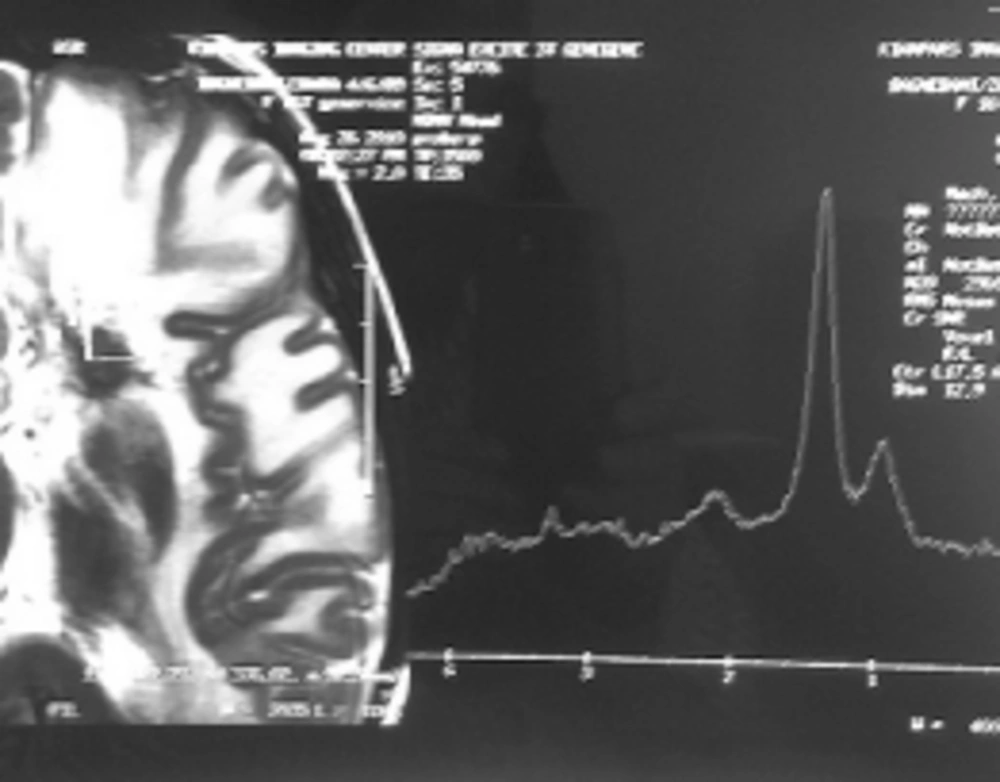
Case 1: A 15-year-old girl with acute lymphoblastic leukemia diagnosed in 2010 was receiving maintenance treatment. Three weeks ago, she referred to a hospital with headache, nausea and vomiting. CT scan of the brain demonstrated low density of mass like a lesion of the left parietal lobes cortico medullary junction accompanies with mild vasogenic edema and no enhancement after contrast media administration. Findings were non specific in CT scan. MRI was applied and on the obtained images there was left parietal corticomedullary junction intermediate signal intensity lesion with vasogenic edema on T2 which as a bright intermediated signal in T1 and low signal intensity peripheral edema (Figure 1A, 1B), follow in contrast media administration remarkable enhancements of lesions are seen (Figure 1C). These findings were compatible with toxoplasmosis and lymphoma. MRI spectroscopy (MRS) was done and increasing of lactate and lipid and low choline were seen in the spectrum of MRS which are specific for necrotizing abscess (Figure 2). Ig M antibody against Toxoplasma by Chemiluminesans (Roche kit) was 8 IU/mL (reference range: non reactive < 0.8; intermitant 0.8- 1; reactive > 1) and Ig G was 4 IU/mL (reference range: Non reactive ˂ 1; intermitant 1- 3; reactive ˃ 3). The patient prescribed with pyrimethamin, sulfadiazine and leucoverin.
The mass was operated and sample sent to pathology department. Histopathological examination revealed necrotic inflammatory lesion, Ziehl- Nelson and PAS staining were negative for acid- fast bacilli and fungi, compatible with toxoplasmosis. The patient expired eight months after toxoplasmosis infection with bone marrow relapse in re- induction period of chemotherapy.
A: T1- Weighted axial MRI: Left parietal corticomedullary junction, bright intermediated signal and low signal intensity peripheral edema. B: T2- Weighted axial MRI: Left parietal corticomedullary junction, intermediate signal intensity lesion with vasogenic edema. C: MRI with contrast shows remarkable enhancement of lesions.
Case 2: A six-year-old boy with acute myeloblastic leukemia (AML) diagnosed in 2010 and was on maintenance treatment. Two months ago he was admitted to a hospital because of fever. Administration of antibacterial, antifungal and antiviral medicine did not control the temperature and due to generalized status tonic clonic convulsion, patient transferred into the intensive care unit (ICU).
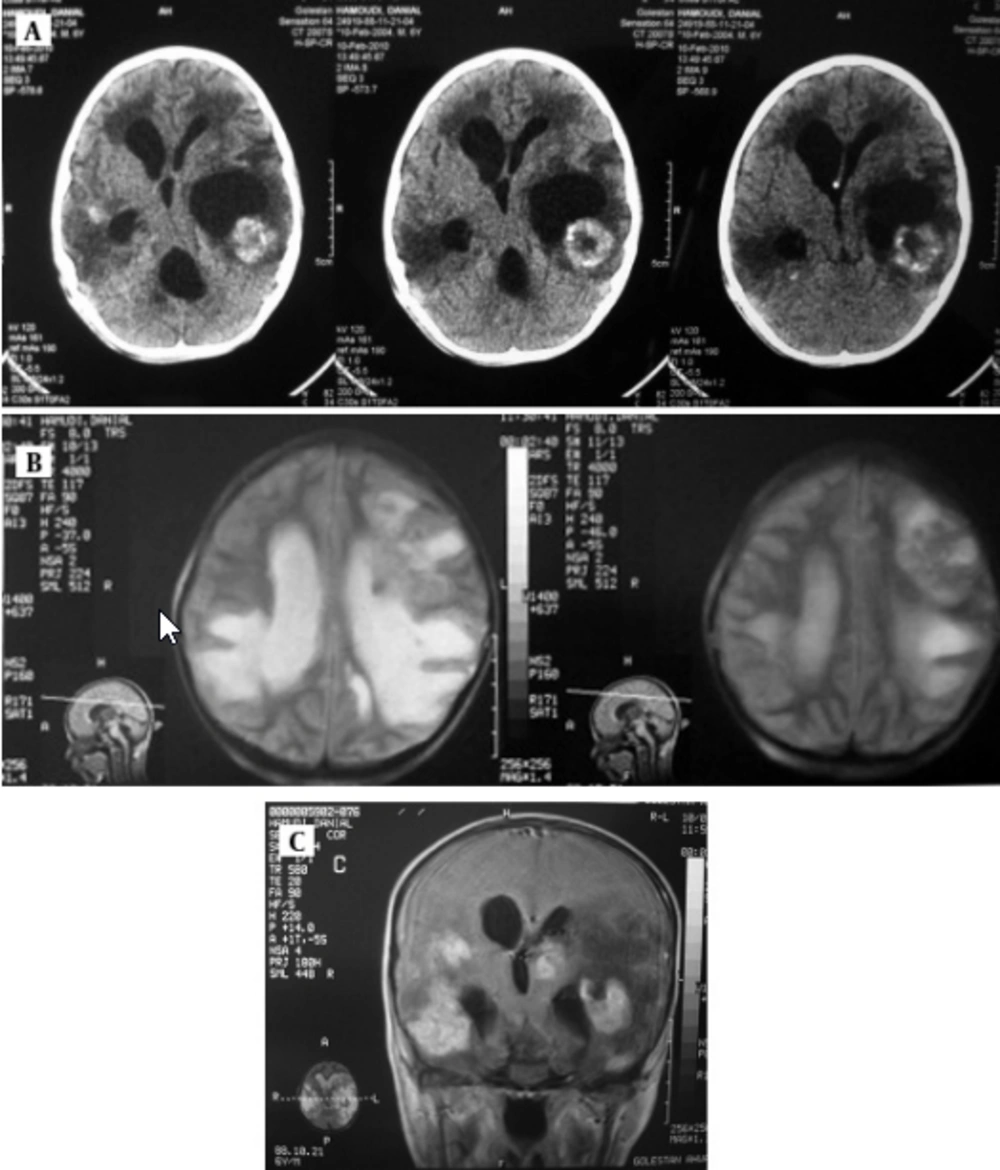
Serological examination for anti- Toxoplasma antibody (Ig M) rate by Chemiluminesans (Roche kit) was more than 9 and Ig G was 6 IU/mL. Brain MRI revealed marked communicating hydrocephaly with corticomedullary junction of both temporal. Hyperdense lesions on T1 with surrounding vasogenic edema and intermediate signal on T2 with bright signal vasogenic edema and on post contrast images multiple enhancing lesions were seen (Figure 3). The patient received pyrimethamin, sulfadiazine and leucovorin. The patient is alive and has neurogic sequel.
3. Conclusions
T. gondii infections in most of the immune- competent humans are asymptomatic, but can cause acute infection in immunosuppressed patients and congenital toxoplasmosis. It has been known that 15- 58% of humans are infected with T. gondii, but the rate of infection varies due to many factors (8). Disease in immune- compromised individuals (i.e. persons with AIDS, transplant recipients, immunosuppressive drug users) usually due to reactivation of latent infection and can lead to lethal meningoencephalitis, focal lesion of CNS and less commonly, myocarditis or pneumonitis. The clinical pictures of cerebral toxoplasmosis may include headache, seizures, mental status changes, focal neurologic signs and aseptic meningitis (9).
In this presentation, two patients with ALL and AML under maintenance treatment were suffering from cerebral toxoplasmosis confirmed by determination of Ig M antibody against Toxoplasma and MRI. Both patients were using maintenance treatment for more than one year and chemotherapy cause the weakening of immune system and reactivation of latent infection. The first patient expired eight months after toxoplasmosis infection, and the second patient is still alive with the sequel. Ghasemian et al. in 2007 evaluated the anti Toxoplasma antibodies (Ig G and Ig M) in 252 cancer patients in Ahvaz and indicated that 15% of the cases were Ig G positive and 10.3% were positive for anti- Toxoplasma Ig M and 6.7% of cases revealed seropositivity for Ig G and Ig M antibodies (10).
Toxoplasmosis has been reported in immune- compromised patients with lymphatic leukemia (11, 12), non- hodgkine lymphoma (13), bone marrow transplantation (14), AIDS (15, 16), and means that if the immune system declines, the tissue cysts may reactivate and cause disseminated infection that manifests as encephalitis, myocarditis or chorioretinitis and can be fetal for immune- compromised patients (17, 18). Although imaging usually shows the hypointensity lesion on T2 and hyperintensive on T1, but Toxoplasma PCR is a useful technique for diagnosis of toxoplasmosis in blood and aqueous humor in patients with HIV, chorioretinitis, pulmonary, cerebral and congenital toxoplasmosis (19, 20). Disseminated toxoplasmosis is a major health problem in immune- compromised patients and it is necessary to examine the patients before, during and after chemotherapy for toxoplasmosis.