1. Background
Nano (according to Merriam-Webster Dictionary) meansone-billionth part of a substance. The high surface area to volume ratio of nanoparticles makes nanoparticles very reactive or catalytic (1). Synthesis of nanoparticles by physical methods usually requires high operating temperatures or pressures, such as production of ZnO (zinc oxide) nano-particles by injection of heat and hydrothermal method or synthesis of ZnS (zinc sulphide) by ultrasonic (2, 3). Chemical methods for synthesis of zinc nano-particles similar to zinc acetate solution, spray pyrolysis method by using a solution of zinc acetate and pressure, or production of Zn and ZnO nanoparticles by chemical vapor deposition with laser create problems and contamination (4, 5). It is necessary to develop, clean, nontoxic synthesis methods compatible with Eco (Green Chemistry). Recent developments have used microorganisms for the synthesis of nanoparticles (6-10). Undoubtedly, nanoparticles have an important role in the future technology. Zinc, zinc oxide and zinc sulfide nanoparticles are used for various biomedical reasons due to their biological characteristics. For instance, ZnS nanoparticles are used as fluorescent probes and for optical imaging labeling of biological tissues. Routinely, magnetic nanoparticles are used as factors for increasing MRI contrast. Designing and modifying the surface of ZnO nanoparticles are used for destruction of tumor cells. Drug delivery is another application of zinc oxide nanoparticles (11-14).
Nano zinc oxide is useful in oil, gas and petrochemical industries as a catalyst. It is also applied for producing rubber, glazing, electronics, cosmetic, and in absorption towers, as well as acting as a catalyst for conventional petrochemical industries and for absorbing ammonia gas. Zinc oxide is used as a highly selective sensor for ammonia gas detection. Also it is useful for H2S removal from drilling fluid (15, 16). Zinc oxide nanoparticles are used for production of antimicrobial fabrics (17).
Microorganisms are used as factories for producing 1-200 nm nanoparticles at room temperature or higher temperatures (in thermophiles) using cheap, renewable carbon sources such as fiber or starch. Microbial resistance against heavy metal ions has been exploited for biological metal recovery via reduction of metal ions or formation of metal sulfides. Most metal ions are toxic for bacteria so reducing ions and forming water from soluble complexes,results a defense mechanism to overcome such toxicity in bacteria (18, 19). Most microorganisms produce their nano-materials by bio-mineralization. Pseudomonas stutzeri is a Gram-negative, rod-shaped, motile, single polar-flagellated, soil bacterium first isolated from human spinal fluid. The production of silver nanoparticles and zinc sulphate with a range of 10-20 nm have been reported for P. stutzeri and Streptomyces sp. HBUM171191 (20, 21).
P. stutzeri contains the CzcC gene. The PSTAB_1242 cobalt-zinc-cadmium resistance protein (CzcC) gene sequence from P. stutzeri was obtained from the National Center for Biotechnology Information (NCBI). This gene is a metal resistance gene that transports Zinc ions across the cytoplasmic membrane and thus may be involved in producing nano zinc.
2. Objectives
The aim of this study was to produce nanoparticles such as ZnO, ZnS, and Zn by using biofilm of a resistant bacterium (i.e. P. stutzeri) on a piece of pure zinc. Optimization of environmental conditions for production of nanoparticles, their biocide effects as well as magnetic properties were other goals of this investigation. Finally, a PCR reaction for the specific PST gene was done. To the best of our knowledge, there are no reports for nano zinc production by bacteria.
3. Materials and Methods
Previously, P. stutzeri strain CS-2 had been isolated from methyl tertiary butyl ether (MTBE) enriched soil and identified by PCR of 16srRNA with two forward and reverses universal primers. This isolate was submitted to NCBI with accession number of FM 957535.1 GI: 220980578.
3.1. Nano Zn Production by P. stutzeri
P. stutzeri was cultured in nutrient agar and incubated for 2 days at 27 °C. Then, one piece of sterile Zn metal sized 3.1 cm was put on the culture and incubated for 2 weeks at 27 °C. Yellowish-white bio-film produced on the piece of zinc metal was separated and dried at 37 °C. The dried biofilm was sent to the Central Lab of Isfahan University and studied by X-Ray diffractometer (XRD, Instrument Specifications: Bruker, D8ADVANCE, Germany, X-Ray Tube Anode: Cu, Wavelength: 1.5406 Å (Cu Kα), Filter: Ni). The percentage and size of the nanoparticles were determined by XRD. Also, P. stutzeri was cultured in two flasks of nutrient broth containing a piece of sterile zinc metal sized 3.1 cm. One flask was incubatedat aerobic and the other at anaerobic conditions for 2 weeks at 27 °C; pH = 7 for the aerobic condition and, KOH was added to obtain pH= 12 for the anaerobic condition that was filled with paraffin. After incubation, the nutrient broth medium treated at the aerobic condition was centrifuged at 13000 rpm for 10 minutes. The supernatant and sedimentation of cells were dried at 37 °C and the samples were analyzed by XRD. Also the dried biofilm produced from the anaerobic situation was analyzed by XRD.
3.2. Production of Nano ZnS
P. stutzeri was cultured in a nutrient broth and nutrient agar containing Na2S 0.04 M in aerobic and anaerobic condition at 27 °C. The sterile zinc metal piece was put in the above mentioned media and the effect of Na2S in production of ZnS was investigated by XRD.
3.3. The Effect of Environmental Condition on Nano ZnO Production
The P. stutzeri was grown on nutrient broth and the effects of various pH (4, 7 and 11.5), light (on and off), and temperatures (23 °C and 37 °C) were studied to determine the optimal condition required for the production of nano ZnO.
3.4. The Antibacterial Activity of ZnO
The antibacterial activity of ZnO produced by P. stutzeri was assayed on nutrient agar against Escherichia coli, Staphylococcus aureus, Brevundimonas diminuta and P.stutzeri by disc diffusion in lawn culture of microorganisms by the Kirby-Bauer disk diffusion method (22).
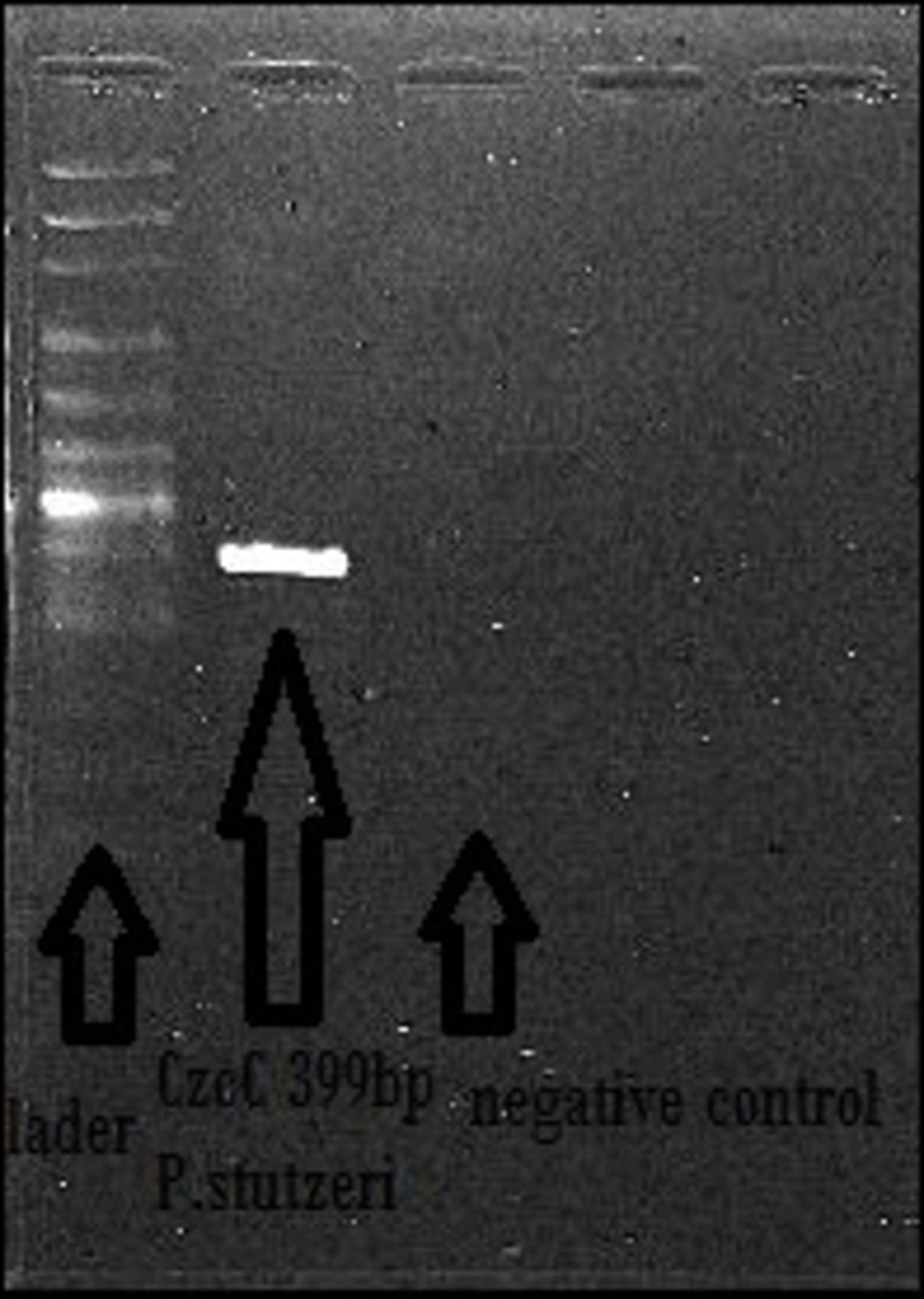
3.5. PCR Reaction of PST Gene
Two primers, Forward 5΄CGAGCGGGCTGCTAAGGTCG3΄ and Reverse 5΄ACGAGGTTGACGCGCTCACG3΄ were designated for the PSTAB-1242 cobalt-zinc-cadmium resistance protein CzcC gene and PCR reaction was performed at the following condition: denaturing at 94 °C for 45 seconds, annealing at 55 °C for 60 seconds and extension at 72 °C for 90 seconds.
4. Results
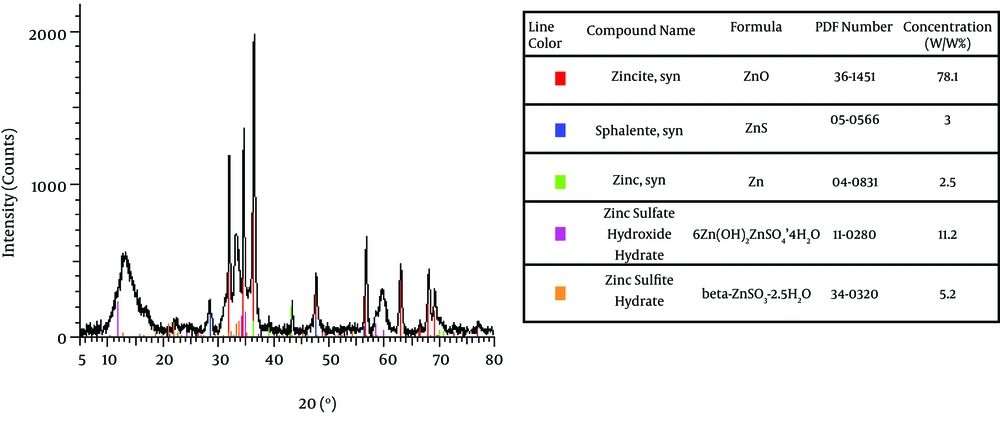
The resistant P. stutzeri isolated from enriched soil containing MTBE was a Gram negative, fast growing bacterium with yellow pigment. Inoculation of this strain in nutrient agar with sterilized zinc metal produced a biofilm colored yellow and white on zinc metal. The obtained data by XRD is shown in Figure 1. As demonstrated, this bacterium produced nano size particles of ZnO (sized 16.35 nm), ZnS (sized 12 nm), and Zn (sized 44 nm). Most of the nano Zn produced was nano ZnO. The percentage of nano particles was assayed by XRD. Results showed that the produced nano ZnO, nano ZnS, and nano Zn were 78.7%, 3% and 2.5% respectively. Nonanoparticle was detected by XRD analyses in the supernatant showing that nanoparticles are on cell surfaces of bacteria.
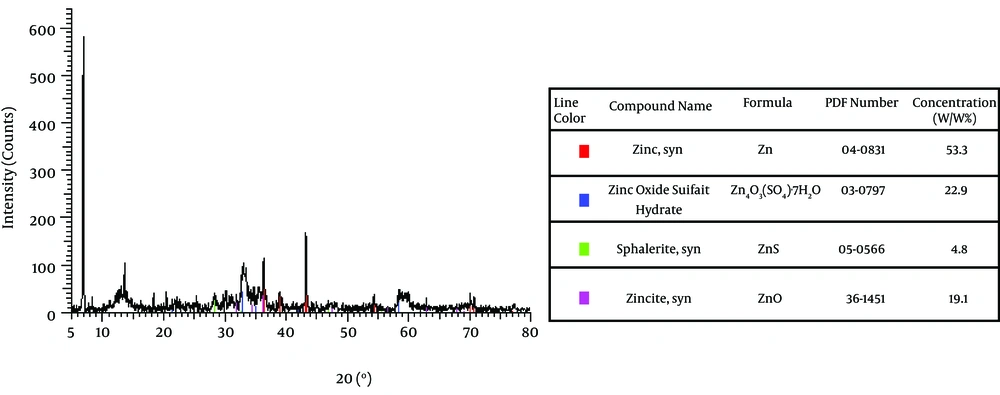
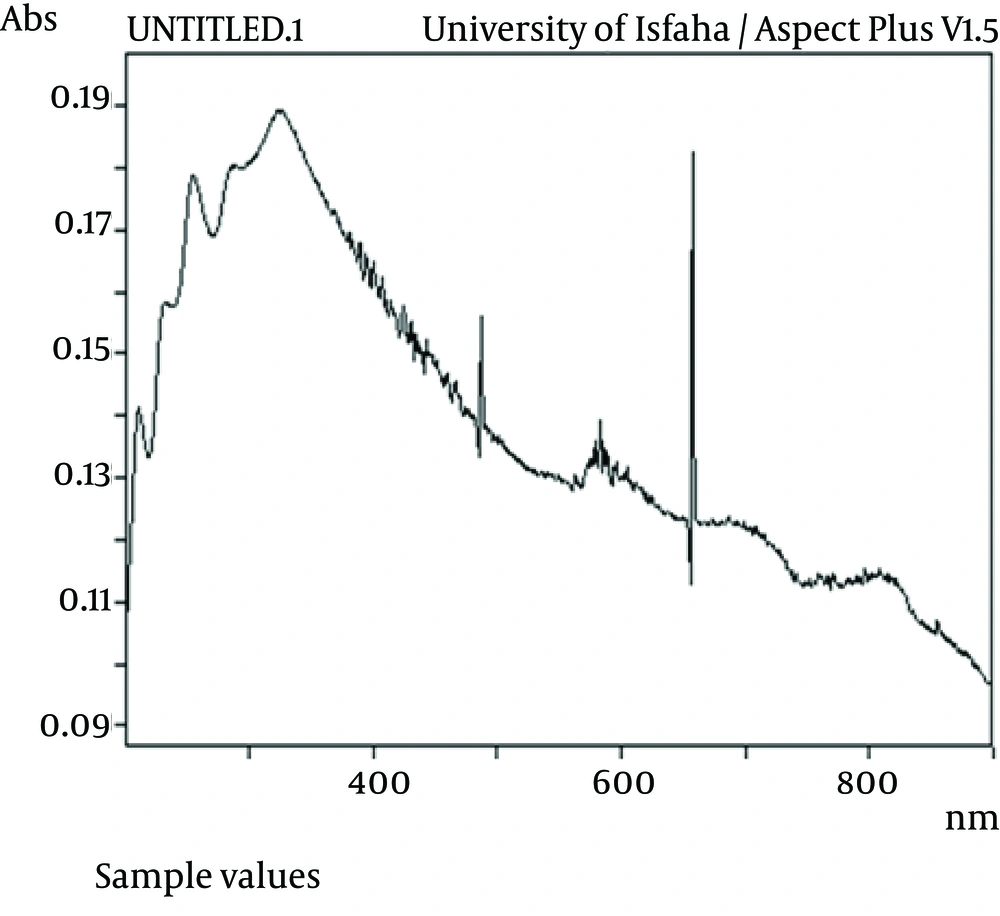
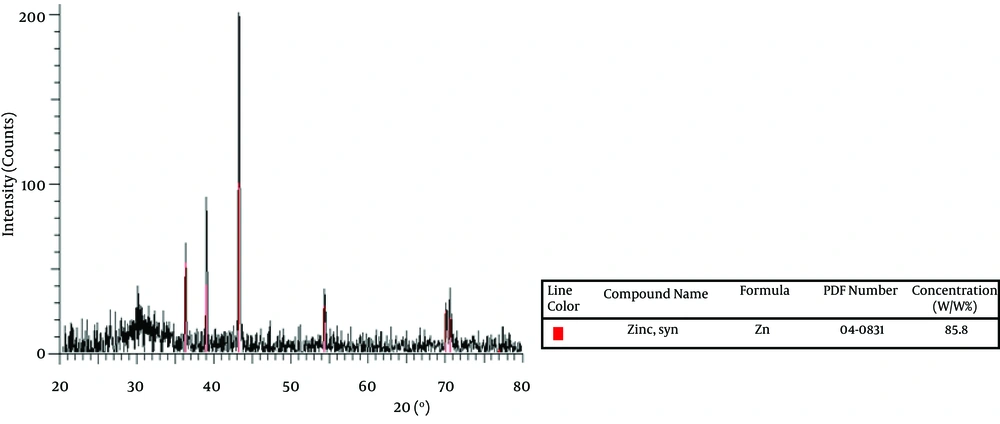
The effect of Na 2 S on ZnS production is shown in Figure 2. As demonstrated, the percentage of zinc oxide sulfate produced was 22.9% while this was lower forZnS (4.8%). Also UV adsorption of ZnS at 340 nm indicates the production of ZnS, which is shown by Figure 3. On the other hand, amount of produced ZnS was higher in aerobic conditions at PH=12. The highest yield was for nano Zn in aerobic condition i.e. 85.8% as shown by Figure 4. Mixed nanoparticles produced by P. stutzeri showed magnetite properties under aerobic and anaerobic conditions. The best condition for producing nano zinc was pH =7, in the light at 37 °C.
| Bacteria | Zone of Inhibition (mm) |
|---|---|
| Escherichia coli | 10 |
| Staphylococcus aureus | 4 |
| Pseudomonas stuzeri | 22 |
| Brevundimonas diminuta | -- |
The PCR reaction of CzcC gene was done for P. stutzeri. As indicated by Figure 5, CzcC gene has a size of 399 Kb. Our results suggest that the isolated strain contains a resistance gene to Zinc with the ability of producing zinc nanoparticles. The antibacterial activity of nano ZnO was detected against E. coli and S. aureus, as shown in Table 1. The highest activity was seen against P. stutzeri with zone of inhibition of 22 mm. No antibacterial activity was detected against B. diminuta.
Overall, in this study, pure culture of P. stutzeri was able to produce nano Zn, nano ZnO, nano ZnS and etc.,as indicated by Figure 1 and Figure 2. The presence of specific genes such as CzcC in P. stutzeri was confirmed, as a gene involved in resistant to zinc. Also, magnetite properties of the produced nanoparticles, the effect of their toxicity on other bacteria and the best condition for producing zinc were assayed.
5. Discussion
Zinc, zinc oxide and zinc sulfide nanoparticles are used for various biomedical, petrochemical and antibacterial applications due to their reactive characteristics (11-17). Synthesis of nano ZnO, nano ZnS and nano Zn is done by physical and chemical methods, which require high temperatures or pressure. Also, their production causes lots of problems as well as contamination (2-5). Thus, their synthesis needs to be substituted by biological methods. The advantages of biological assays are that they are nontoxic, clean, cost effective and compatible with Eco (Green Chemistry).
In this study, a resistant bacterium i.e. P. stutzeri was a good candidate for producing zinc nanoparticles. The reasons that P. stutzeri was used in this study, was the presence of metal resistance genes in this bacterium. Different types of zinc nanoparticles were produced by P. stutzeri. By optimizing the environmental conditions, the highest yield was gained for zinc oxide (78.1%). There are a few reports regarding the anti-bacterial activity of zinc oxide nanoparticles on Gram positive and negative bacteria (17, 23). It needs to be mentioned that previous reports on producing ZnOnanoparticles were prepared by wet chemical methods which were directly applied on to the 100% cotton woven fabric (17). Their results showed that the finished fabric confirmed considerable anti-bacterial activity against S. aureus in both qualitative and quantitative tests (17).
Iron is particularly important in magnetotactic bacteria because these microorganisms incorporate large amounts of iron in the form of nano magnetite iron mineral crystals within their cells (24). The magnetic property of ferrite zinc formed by a microbial process was shown previously by chemical methods. A metal reducing bacterium, thermoanaerobacter, has been shown to produce nanoparticulates of ferrite zinc (25). In the present work, the produced zinc nanoparticles had magnetite properties in the vicinity of the bacterial cells that might be a result of cytochrome or other Fe proteins, that conjugate with nano zinc and magnetite produced particles.
The most important reason for choosing P.stuteri for producing zinc nanoparticles was the resistance gene in this bacterium. The specific primers for zinc resistance CzcC gene was designed and P.stuteri was amplified by PCR and the results showed that the gene was present in the isolate, so it is probably that the zinc resistant microorganism is able to produce nano zinc particles too. This gene was also observed on other bacteria (26, 27).
To the best of our knowledge, production of zinc nanoparticles including zinc oxide nanoparticles, nano zinc sulfide, and nano zinc has not been reported for any microorganisms while, recently, production of zinc sulfate nanoparticles, has been reported for a species of Streptomyces (21). Although in previous studies it was shown that P. stutzeri is able to produce silver nanoparticles in a silver salt solution (20), but in this study, for the first time ZnO, ZnS, and Zn nanoparticles were detected on a biofilm of zinc metal.