1. Background
Hepatitis B virus (HBV) infection is a major public health problem worldwide. It is estimated that more than 400 million of the world population are infected with HBV resulting in 600000 to one million deaths annually (1, 2). Chronic HBV may lead to cirrhosis, fibrosis and hepatocellular carcinoma (3) In Asia, the majority of HBV infections are acquired parentally or, in early childhood, through vertical (mother-to-child) transmission; however, horizontal transmission is the main route of infection in African and Western countries (2). The prevalence of HBV Infection among the different groups of patients was also reported in Iran (4-7). So far 10 HBV genotypes (A-J) and different sub-genotypes have been reported (8). HBV genotypes A and D are prevalent in Western countries and India, B and C in Asia, genotype E in west Africa, genotype F in central America, G in the USA and recently HBV genotypes I and J were reported in Vietnam and Japan respectively (9-12).
Occult hepatitis B is one of the mysterious and important kinds of hepatitis B infection, but there are different definitions of the occult HBV infection (OBI). Raimondo et al. (13) defined OBI as the presence of HBV DNA in the liver (with detectable or undetectable HBV DNA in the serum) of individuals that are negative for HBsAg by serological tests. OBI may be distinguished as seropositive-OBI (anti-HBc and/or anti-HBs positive) and seronegative-OBI (anti-HBc and anti-HBs negative) (13). Briefly, occult HBV infection was defined as the presence of HBV DNA in the sera or in the liver biopsy and the absence of HBsAg by conventional serological tests (14-16). The occult HBV was reported in patients with raised ALT (17). In the endemic regions of HBV infection, patients are more likely to develop occult HBV infections (18).
Occult HBV infection could be transmitted via several routs including blood transfusion and organ transplantation (19). Moreover, OBI may progress to liver fibrosis and hepatocellular carcinoma (20). The prevalence of occult hepatitis B infection varies from less than 1% in Hong Kong to as high as 87% in Japan (21). There are several reports about occult hepatitis B prevalence in Iran. The study conducted by Shavakhi et al. (22) showed the prevalence of 19.4% occult hepatitis B infection in patients with hepatitis C infection. Keyvani et al. (23) detected 4.9% occult hepatitis B cases in hemodialysis patients in Tehran, and Arababadi et al. (24) reported 16.1% occult hepatitis B among the blood donors in Rafsanjan. The present study was undertaken to investigate the prevalence of occult HBV infection in the patients with abnormal ALT in Ahvaz city, Iran. Ahvaz city is a center of Khuzestan province, with population of 1.5 million, in Southwestern Iran.
2. Objectives
The current study aimed to evaluate the occult HBV infection by PCR and determine HBV genotyping among the patients with abnormal alanine transaminase (ALT) in Ahvaz city, Iran.
3. Patients and Methods
The current cross sectional study was carried out on 120 patients with abnormal ALT and AST (aspartate aminotransferase), including 54 (45%) females and 66 (55%) males from different region of Khuzestan province who attended Danesh Medical Laboratory in Ahvaz during February 2011 to February 2012 (Table 1). All the patients had abnormal ALT 40-152 IU/L (mean 58 ± 12 IU/L) and AST 11-102 IU/L (mean 34 ± 16) for more than one year. The collected sera were included in this study after receiving patients` written consent. The patients` age was from 4 to 72 with the mean of 48 ± 14 years (Table 2). All the patients’ sera were found negative for HCV antibody, HCV RNA, HBs Ag, HEV IgM and HAV IgM by ELISA test (Diapro Co., Italy). Other serological markers including HBcIgG (Diapro Co., Italy) were carried out for all sera. Patients with fatty liver disease, drug users, autoimmune hepatitis, genetic disease and metabolic disorders were excluded.
| Gender | HBV Positive | HBV Negative | Total |
|---|---|---|---|
| Male | 6 (11.1) | 48 (88.9) | 54 (100) |
| Female | 6 (9.1) | 60 (90.9) | 66 (100) |
| Total | 12 (10) | 108 (90) | 120 (100) |
aData are presented as No. (%).
| Age group | HBV Positive | HBV Negative | Total |
|---|---|---|---|
| Unknown | 3 (18) | 13 (82) | 16 (100) |
| Less than 20 | 1 (16.7) | 5 (83.3) | 6 (100) |
| 21 up to 40 | 4 (8.3) | 44 (91.7) | 48 (100) |
| 41 up to 60 | 4 (8.8) | 41 (91.2) | 45 (100) |
| More than 60 | 0 | 5 (100) | 5 (100) |
| Total | 12 (10) | 108 (90) | 120 (100) |
aData are presented as No. (%).
3.1. DNA Extraction
Viral nucleic acid was extracted from 200 µL of each patient’s serum using the high pure viral nucleic acid kit (Roche Applied Science, Germany) according to the manufacturer`s instructions.
3.2. Polymerase Chain Reaction of Surface Gene Amplification
The specific primers for the partial S region of HBV (Bioneer company, Korea) were used for the nested PCR (25).
FHBS1: 5'-GAGTCTAGACTCGTGGTGGACTTC-3'
RHBS1: 5'-CGTGGTGGACTTCTC TCAATTTTC-3'
FHBS2: 5'-AAA TKGCACTAGTAAACTGAGCCA-3'
RHBS2: 5'-GCCARGAGAAACGGRCTGAGGCCC-3'
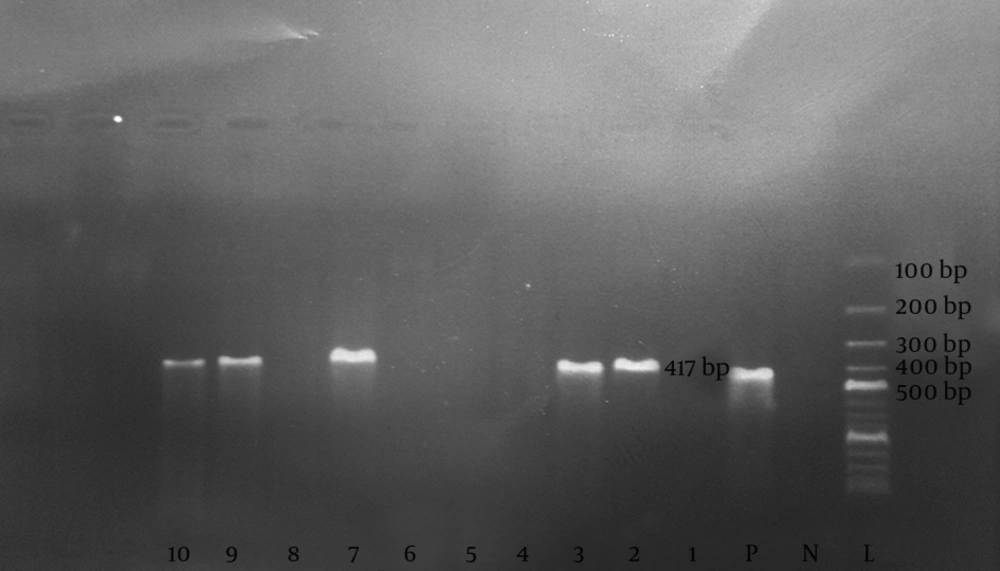
The primer position of the S region in the HBV genome were as follows: FHBS1 (244 to 267), FHBS2 (255 to 278), R HBS2 (648 to 671), and R HBS1 (668 to 691) (19). Initially, 5 µL of the extracted DNA from each serum sample was added on PCR reaction mixture containing dNTP (10 Mm) 0.5 µL (Roche Co., Germany), PCR buffer (10x) 2.5 µL (Roche Co., Germany), Taq DNA polymerase 5U (Roche Co., Germany) 0.15 µL, 50 pmol/µL of both FHBS1 and RHBS1 primers and Distilled water 15.85 µL. The mixture was put in the thermocycler apparatus (Techne Company, UK) and the first amplification was carried out with initial denaturation at 94°C for 5 minutes, followed by denaturation at 94°C for 30 seconds, annealing at 56°C for 30 seconds, extension at 72°C for 30 seconds for a total of 30 cycles. For the second run, 5 µL of each PCR product was added on 20 µL reaction mixture containing dNTP, PCR buffer and Taq DNA polymerase with 50 pmol/µL of each FHBS2 and RHBS2 primers. The amplification was carried out using termocycler with the same program of the first run. The 8 µL of the second run PCR product (417 base pare) was analyzed by the 2% agarose gel (CinaGen Co., Iran) electrophoresis containing safe stain (CinaGen Co., Iran) (Figure 1).
3.3. Sequencing and Phylogenic Analysis
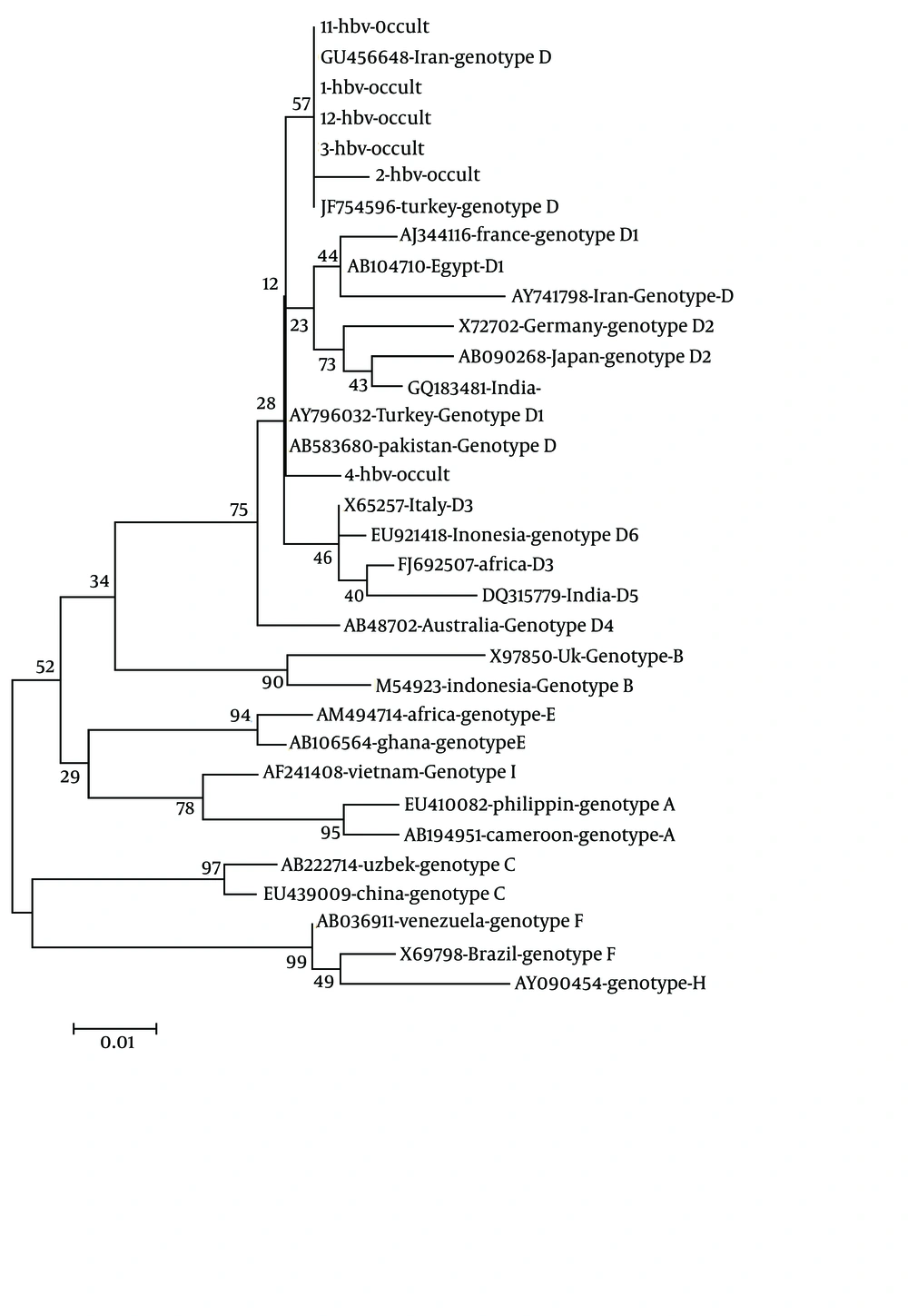
The positive PCR products were sent to Bioneer Company (Korea) and sequenced in one direction to determine HBV genotyping. The analysis of phylogenic tree of the partial S region was carried out by neighbor-joining method under the Kimura two-parameter distance model (Figure 2). The MEGA software version 4 (www.megasoftware.net) was employed to implement the methods.
3.4. Statistical Analysis
The SPSS software version 14.0 and chi-square test were used for data analysis. The significance level was P < 0.05.
4. Results
Out of 120 sera, 12 (10%) sera, including six (5%) males and six (5%) females, were positive for HBV DNA by PCR (P > 0.05). Of these 12 patients seven (5.83%) were positive for HBcIgG. The prevalence of HBcIgG among the two genders was three (2.5%) in male and four (3.3%) in female patients (P > 0.05). The frequency of OBI among the age groups was three (2.5%) cases in 20-29 years, three (2.5%) cases in 30-39 years, two (1.65%) cases in 40-49 years, and four (3.3%) cases in 50-59 years. The sequencing result of the PCR product from the six patients including three male and three female revealed that only genotype D was predominant with one D1 subtype among the (OBI) patients.
5. Discussion
Investigation of possible OBI should be done for those patients with abnormal ALT lasting for years. Under such circumstances hepatitis B virus could remain and replicate in patients` liver and transmit to people via different routes. Detection of occult hepatitis B in patients can help to increase knowledge and prevent virus transmission. Occult HBV infection is being recognized in many parts of the world, especially in countries with intermediate to high endemicity. Point mutations, deletions and integration of HBV DNA cause some amino acid changes in HBsAg which can cause failure in HBsAg detection (17).
Occult HBV is recognized as HBsAg negative, with the presence of HBV DNA in liver with or without DNA in serum and with or without anti-HBc IgG. Some cases with anti-HBs or anti-HBc positive are recognized as seropositive occult hepatitis B (13, 26, 27). In the present study, about 5.83% of the patients were positive for HBcIgG without significant relationship between HBcIgG positivity and gender (P > 0.05). The prevalence of OBI varies from 1% to 87% in different geographical regions (21). Occult HBV infection with measured ALT has been reported 19.4%, 4.9%, and 16.1% among the different high risk groups in Iran (22-24). These reports are comparable with those of the current study results (10%) and emphasize on the variety of OBI prevalence based on mentioned geographical regions.
The prevalence of the occult HBV infection among the patients with measued ALT was reported 28% in India (28). In the current study, all the 12 OBI patients had raised abnormal liver ALT; however liver biopsy was not performed on them. The prevalence of the occult HBV infection among the males and females was insignificant (P > 0.05). In the current study the phylogenic comparison revealed that all the OBI had predominant genotype D and only one sub genotype D1. The D2 sub-genotype and G, A1, D1 and D5 sub-genotypes have been already reported from India (28, 29). The Genotype D has been already reported in Iran (30); HBV genotype D is predominant among the Iranian neighboring countries including Afghanistan (31), UAE (32), and Pakistan (33). The remaining 108 (90%) patients were negative for occult HBV infection; however the role of occult hepatitis C virus, which requires further investigation, was not evaluated in the present study (34).