1. Background
Pneumocystisjiroveci is an important determinant of mortality in immunocompromised patients specially AIDS patients (1). Regardless of the source of infection, Pneumocystis can survive and multiply for a long time after entering the body without causing clinical symptoms. After some time, by induction of the immune system of the healthy individual against the organism, multiplication of the infectious agent is controlled, causing infection without symptoms (2). Pneumocystis will evade the immune system surveillance in immunocompromised patients, causing disease symptoms by increasing multiplication (3).
Staining methods and microscopic observation have been considered as the gold standard for diagnosis of Pneumocystis, worldwide (4). One of the disadvantages of microscopic diagnosis is that the patient must be in the final phase of the disease, such that the number of organisms in respiratory samples must reach a threshold to be visible and diagnosed (5). Bronchoalveolar lavage (BAL) specimens have the highest sensitivity among non-invasive samples in detection of P.jiroveci pneumonia (PJP) (6). P.jiroveci pneumonia can be controlled and treated in its early stages. Therefore, quick and timely detection of latent Pneumocystis in susceptible patients is very important for clinicians (7). The use of molecular methods can play an important role in identification and biological control of pneumocystosis (8). The first lung transplant in Iran was performed in 2001, after which many patients have received a lung transplant in this country (9). Lung transplant recipients are susceptible to develop a range of opportunistic infectious diseases. Screening for opportunistic infectious agents in transplant recipients can be directly correlated with patient's health (10, 11).
2. Objectives
Given the importance of molecular epidemiology studies, this study aimed to determine the colonization of Pneumocystis among lung transplant patients undergoing bronchoscopy.
3. Patients and Methods
3.1. Patients
This cross sectional study was conducted on 32 BAL specimens of lung transplant recipients undergoing bronchoscopy under a physician's orders due to respiratory problems. Samples were collected from several specialized hospitals in Tehran from September 2010 to October 2012, after obtaining an informed consent from both the patients and physicians. All the patients were negative for tuberculosis and HIV.
3.2. DNA Preparation and Extraction
Bronchoalveolar lavage specimens were collected in sterile conditions and homogenized by 10 mM dithiotritol (12). Genomic DNA was extracted using the MTB extraction kit manufactured by Roche.
3.3. Primers
Genome amplification was done using initial primers pAZ102-E: 5'-GATGGCTGTTTCCAAGCCCA-3' and pAZ102-H: 5'-GTGTACGTTGCAAAGTACTC-3' (13). Next, the initial PCR product was amplified by internal primers pAZ102-E and pAZ102-L2: 5'-ATAAGGTAGATAGTCGAAAG-3' (14).
3.4. PCR
Polymerase chain reaction was performed in a final volume of 25 μL by adding 1 μL of 10 pM primers, 0.8 μL magnesium chloride, 0.8 μL dNTP, 0.8 μL Taq DNA, 2.5 μL of PCR buffer and 2.5 μL DNA template. The thermal protocol of the PCR was as follows: four minutes of initial denaturation, 30 seconds for denaturation of DNA strands, primer binding for initial PCR for 30 seconds at 59.5°C and 56°C for the secondary amplification, 30 seconds at 72°C for extension in 35 cycles, four minutes at 72°C for final extension. The amplified PCR product was run on 1.5% electrophoresis on gel, and was assessed in the transilluminator device after staining with ethidium bromide.
3.5. Negative and Positive Controls
In the present study, the positive control was a sample of P.jiroveci isolated and sequenced from HIV positive patients in Iran with code number JF733748, listed on the World Gene Bank (15). Deionized distilled water was used instead of a template as the negative control.
3.6. Statistical Test
Kappa test was used to measure the agreement between PCR and nested PCR. Fisher’s exact test was used to assess the significance between gender, duration of lung transplant and presence of P.jiroveci DNA. Independent t-test was used to assess the significance between age and presence of P.jiroveci DNA. All differences were deemed significant at the level of P < 0.05. Confidence interval of 95% was considered for all tests. All statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 18.0 software (Chicago, Inc., USA).
4. Results
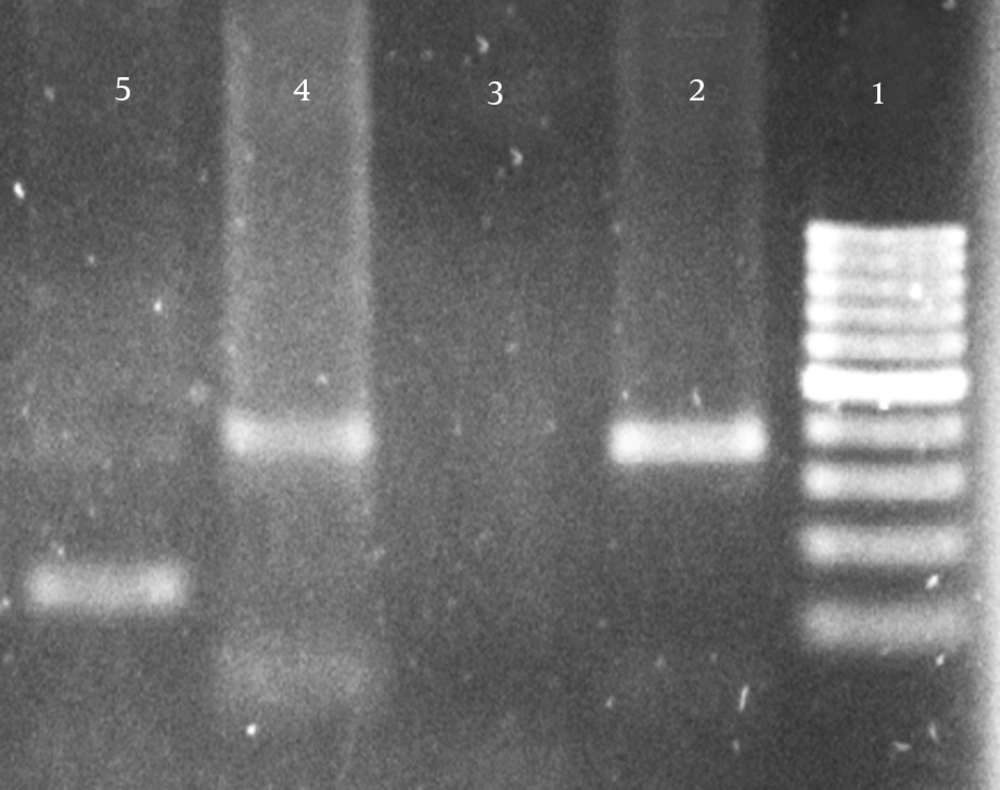
Of the 32 patients under study, there were 24 (75%) males and eight (25%) were females. The average age of patients was 32 ± 8.5 years. Pneumocystis specific DNA amplification was performed, using Paz-102E and Paz-102H primers on 32 BAL specimens of lung transplant patients, and had positive results for four patients (12.5%). The next step was specific genome amplification of P.jiroveci using Paz-102E and Paz-L2 primers from the initial PCR product. Seven samples (21.9%) were positive for P.jiroveci in BAL specimens (Table 1). Positive samples in 1.5% agarose gel electrophoresis produced a 347 bp band in the initial amplification and 120 bp bands in the second amplification, as indicated by Figure 1.
PCR and nested PCR in all 32 patients enrolled in this study indicated four positive cases of P.jiroveci DNA and 25 negative cases. Based on the kappa test, there was 68% agreement between PCR and nested PCR, which is a fairly good degree of agreement. The mean age of negative and positive patients for Pneumocystis DNA using nested PCR was 31.56 and 33.85 years, respectively. No significant correlation was found between positive nested PCR and the patients’ age, using the independent t-test (P = 0.54). Of the 24 male patients under study, there were five patients (20.8%) positive for the P.jiroveci genome, and 19 patients (79.2%) were negative. Among the eight female patients in the study, two patients (25%) were positive for the P.jiroveci genome and six patients (75%) were negative. The fisher exact test indicated that there was no significant relationship between the presence of P.jiroveci genome and patient’s gender (P = 0.99).
Among the 18 patients who had received a lung transplant within the last six months, four patients (22.2%) were positive for the P.jiroveci genome. From 14 lung transplant patients whose transplant duration was more than six months, three patients (21.4%) were positive for the P.jiroveci genome. Using the fisher’s exact test, there was no significant relationship between duration of lung transplant and presence of P.jiroveci genome (P = 0.99).
| Single Round PCR No. (%) | Nested PCR No. (%) | |||||
|---|---|---|---|---|---|---|
| Transplantation Time | Positive | Negative | Total | Positive | Negative | Total |
| Less than 6 months | 1 (25%) | 17 (60.7%) | 18 (56.2%) | 4 (57.1%) | 14 (56.0%) | 18 (56.2%) |
| More than 6 months | 3 (75%) | 11 (39.3%) | 14 (43.8%) | 3 (42.9%) | 11 (44.0%) | 14 (43.8%) |
| Total | 4 (100%) | 28 (100%) | 32 (100%) | 7 (100%) | 25 (100%) | 32 (100%) |
5. Discussion
Treatment protocol for patients with various malignancies and organ transplant recipients may involve immunosuppressive agents such as corticosteroids, cyclosporine or cyclophosphamide. All these drugs act on both arms of the immune system as immunosuppressive agents (16, 17). Control of Pneumocystis infection in people with healthy immune systems is mainly dependent on T-cell activity, but the role of humoral immunity has been indicated by experimental and clinical data (2, 18). Great deal of studies show that the presence of Pneumocystis can be dangerous in susceptible patients, so that presence of Pneumocystis specific amplicons from oral cavity swabs of adult rats without immunosuppression can predict incidence of P.carinii pneumonia (PCP) after immunosuppression, due to corticosteroid administration (19). The first epidemic of Pneumocystis pneumonia was reported between 1961 and 1963 in two orphanages in Shiraz by Post et al.; from the 168 studied children, 40 died and the presence of Pneumocystis was demonstrated in six patients (12.5%) by autopsy (20). Appropriate prophylaxis against P.jiroveci infection is valuable in susceptible patients. In 1971, Post et al. used sulfadoxine pyrimethamine as a prophylaxis in children at risk of Pneumocystis pneumonia in Shiraz orphanages, and the incidence of disease was decreased to 0% from 25% in the control group (21).
In AIDS patients, the risk of PJP is rapidly increased when the number of CD4 + lymphocytes decreases to 200 × 106 per mL. The incidence of PJP is five times higher in those with a CD4 + lymphocyte count of less than 200 × 106 compared to patients with CD4 + counts higher than this value (22, 23). P.jiroveci mortality in HIV positive patients in Iran has been reported to be up to 26.6% (15). Treatment protocol of P.jiroveci among transplant patients is co-trimoxazole consumption three to six months post-transplant (24). P.jiroveci even colonizes healthy individuals free from any respiratory problems. The incidence of P.jiroveci in respiratory samples of healthy subjects has been reported to be up to 20%. Latent Pneumocystis colonization is directly related to the immune system status of the individual (25). P.jiroveci colonization is higher in HIV negative patients with respiratory problems consuming glucocorticoids (26). There are many reports of PJP among patients receiving kidney, heart, bone marrow and lung transplants (27, 28, 29). Molecular identification of P.jiroveci has been done in respiratory specimens of patients with malignancies and respiratory problems. Molecular screening of P. jiroveci in pulmonary lavage samples of non-HIV infected patients in Iran indicates that P. jiroveci colonization is higher in transplant recipients and cancer patients compared to other patients (30).
PCR based diagnosis of Pneumocystis in respiratory specimens is a valuable tool for screening of this organism. Single step PCR cannot precisely detect the infectious agent in some cases, especially in low template DNA conditions. Therefore, in this study the more sensitive and reliable nested PCR was used due to its ability to amplify very small quantities of DNA (31). In this study, four samples in PCR and four samples in the nested PCR were positive for P. jiroveci. A great deal of studies support the presence of latent P. jiroveci in respiratory specimens of patients with malignancies and respiratory problems. There are evidences of P. jiroveci colonization in respiratory specimens of healthcare staff in contact with PJP (32). Several studies indicate transmission of P. jiroveci by air droplets in humans. P. jiroveci has been isolated from ambient air in rooms of patients with PJP using the PCR method (33). Considering the duration of hospitalization of lung transplant recipients, isolating the air in the rooms of these patients seems to be necessary. According to the results of this study, a remarkable presence of P. jiroveci is evident in respiratory samples of lung transplant patients. Therefore, prophylaxis against Pneumocystis is recommended according to available standards.