1. Background
Hyaluronidases are enzymes that hydrolyze hyaluronic acid (HA) to N-acetyl-D-glucosamine (GlcNAc) and D-glucuronic acid (GlcA) in the body. The function of this enzyme is increasing the permeability of tissue to fluids (1). Hyaluronidase is produced in both prokaryotic and eukaryotic cells (2, 3). The catalytic mechanism of hyaluronidase is unknown (4); the structure and biological activity of hyaluronidase have been intensively studied in different microorganisms such as Streptococcus pyogenes (5), S. pneumoniae (6), Enterococcus (7), Clostridium perfringens (8), S. agalactiae (9), Pseudomonas aeruginosa (10) and Staphylococcus aureus (11).
Hyaluronidase in snake and insect venom is thought to function as a “spreading factor” by degrading the host hyaluronic acid, thus allowing the spread of toxin (12, 13). Therapeutic use of this enzyme is as a facilitating factor in the release of toxins and vaccine injected under the skin. Hyaluronidase has different pharmaceutical and medical applications (14) such as in ophthalmology (15), cancer (16), metastasis (17), tumor suppression (18), and fertilization (19) and also this enzyme can be used as a drug in combination with anesthesia and pain medication.
The hyaluronidase enzyme activity has been assayed by several methods including turbid metric (20), viscometer, microtiter-based enzyme linked immunosorbent assay (ELISA)-like assays (21, 22), colorimetric and fluorimetric assay, each of which have a particular function. For instance, colorimetric assay is used for determination of the serum hyaluronidase activity, fluorimetric assay is utilized for definition of biological samples such as human and rabbit serum hyaluronidase, and turbid metric and viscos metric methods are unsatisfactory in clinical use (23-27).
This enzyme has significant implications in different areas such as medical, diagnostic, treatment, physiologic, pathologic and commercial usage; future research and studying is implicated. It is important to produce a small and stable enzyme with same properties and effects. Therefore, in this study, the hyaluronidase antigenic fragments protein was overexpressed and purified in an appropriate condition then, the enzyme activity was measured using the turbid metric method.
2. Objectives
The main goal of our study was to optimize the production of hyaluronidase and improve the enzymatic properties of this enzyme in a smaller size.
3. Materials and Methods
3.1. Designing, Gene Amplification, Cloning and Expression of Antigenic Fragment
To find out the antigenic region, the sequence of hylA [S. pyogenes, accession number: EU078690.1] was submitted to Emboss antigenic server and the region with high antigenic properties was selected. Primers (CinnaGen, Iran) were designed according to the resulted sequences. Genomic DNA was extracted according to the standard CTAB/NaCl method (28) and PCR (Roche, Germany) was used to synthesize the antigenic fragments of hyaluronidase. Afterwards, the fragment was cloned in pGEX-4T-1 expression vector (Pharmacia, Sweden) and expressed in Escherichia Coli BL21 (Pharmacia, Sweden) (29).
3.2. Optimization of protein Expression and Purification
The expression of hyaluronidase antigenic fragment was optimized using different Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Merck, Germany) concentrations (0.5 and 1 mM), times of induction (two hours, four hours and overnight), temperature (30ºC and 37ºC), culture media [NB (Merck, Germany) 1.5x without glucose containing 100 mg ampicillin (Merck, Germany), NB (Merck, Germany) 1.5x with glucose (Merck, Germany) containing 100 mg ampicillin (Merck, Germany), Luria-Bertani (LB) broth (Merck, Germany) in one liter including 10 g yeast extract (Merck, Germany), 20 g Bacto tryptone broth (Merck, Germany), 0.2% (mass/v) glucose (Merck, Germany), 10 g NaCl (Roche, Germany), 1 g KCl (Roche, Germany), 0.5 g MgCl2 (Merck, Germany), 0.5 g CaCl2 (Merck, Germany), 100 mg ampicillin (Merck, Germany) and LB broth (Merck, Germany), and all the mentioned with the same concentration without glucose (Merck, Germany)] with 0.6-0.8-1 absorbance at 600 nm. The expressed proteins were purified using GST-sepharose column according to the manufacture instruction (Sigma, Germany) (29).
3.3. Determination of Protein Concentration
The quality and quantity of the purified recombinant hyaluronidase proteins were assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis [SDS-PAGE (12%)] with the absorbance of 280 and 260 nm, respectively (28). Proteins concentration was measured using the following formula: Measurement of protein concentration (mg/ mL) = (1.55 × OD 280) - (0.76 × OD 260)
3.4. Hyaluronidase Activity Assay
The enzymatic assay of hyaluronidase antigenic fragments was determined as U/mg using a quantitative turbid metric method based on Dorfman, A (30). In principle, hyaluronic acid as substrate is hydrolyzed and depolymerized by hyaluronidase. Conditions of this method are T = 37°C, pH = 5.9, A 600 nm, light path = 1 cm. Briefly, the recombinant protein was dissolved in 1 mL enzyme buffer (0.02 M phosphate buffer, 0.45% NaCl (Roche, Germany), 0.01% bovine serum albumin (BSA) (Sigma, Germany), pH = 6.8-7.0, mixed with 0.5 mg HA (Sigma, Germany), which was dissolved in 1 mL substrate butter (0.3 M KH2PO4/Na2HPO4 (Merck, Germany), pH = 5.30-5.35). Enzyme digestion was allowed to proceed for 45 minutes at 37°C. Turbidity was generated at the end of the incubation by adding 10 mL acid albumin (Merck, Germany) solution. Optical density at 600 nm was determined exactly five minutes after the addition of acid albumin.
National formulary standard hyaluronidase solution (NF Std) (Sigma, Germany) was used as a standard sample in different concentrations and to establish a standard curve; units of activity were calculated by comparing them to this curve. A standard curve was made using commercial hyaluronidase (Sigma, Germany) with different known concentrations diluted from a stock of 400-1000 mg/mL as follows: 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6.
4. Results
4.1. Designing, Gene Amplification, Cloning and Expression of Antigenic Fragment
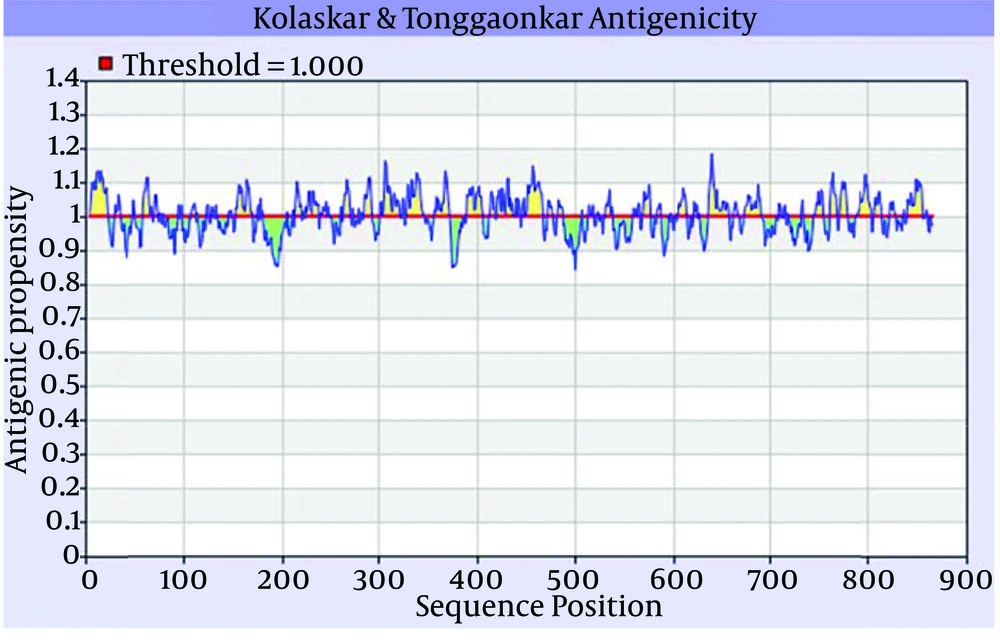
According to the result of the server, the amino acid sequence of 838 till 2320 was selected as a region with high antigenic properties (Figure 1). The target gene was successfully extracted and amplified, then was cloned and expressed in pGEX-4T-1 expression vector (29).
4.2. Optimization of Gene Expression
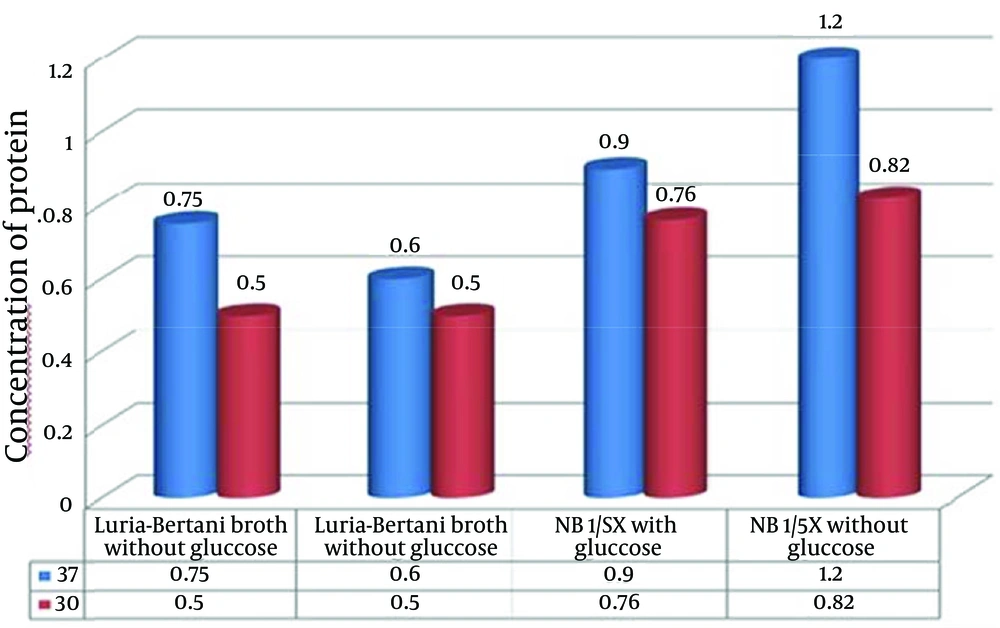
We changed the expression conditions of this protein to obtain the maximum expression. Afterwards, each recombinant protein was purified with affinity chromatography; then, the amount of purified recombinant protein was determined (Table 1).
| Sample | Protein, mg/mL |
|---|---|
| HylA elute sample, 1 mM IPTG and OD = 1 | 0.65 |
| HylA elute sample, 0.5 mM IPTG and OD = 1 | 0.7 |
| HylA elute sample, 1 mM IPTG and OD = 0.8 | 0.85 |
| HylA elute sample, 0.5 mM IPTG and OD = 0.8 | 0.93 |
| HylA elute sample, 1 mM IPTG and OD = 0.6 | 0.47 |
| HylA elute sample, 0.5 mM IPTG and OD = 0.6 | 0.54 |
| HylA elute sample, in NB 1.5x without glucose and induction at 37°C | 1.2 |
| HylA elute sample, in NB 1.5x without glucose and induction at 30°C | 0.82 |
| HylA elute sample, in NB 1.5x with glucose and induction at 37°C | 0.9 |
| HylA elute sample, in NB 1.5x with glucose and induction at 370°C | 0.76 |
| HylA elute sample, in LB broth with glucose and induction at 37°C | 0.6 |
| HylA elute sample, in LB broth with glucose and induction at 30°C | 0.5 |
| HylA elute sample, in LB broth without glucose and induction at 37°C | 0.75 |
| HylA elute sample, in LB broth without glucose and induction at 30°C | 0.5 |
| HylA elute sample, in 2 h of induction and OD = 0.8 | 0.4 |
| HylA elute sample, in 4 h of induction and OD = 0.8 | 0.7 |
| HylA elute sample, in overnight induction and OD = 0.8 | 1.8 |
a Abbreviations: NB, X; OD, optical density; LB, Luria-Bertani; IPTG, Isopropyl β-D-1-thiogalactopyranoside.
4.2.1. Effect of Glucose on HylA Production
To evaluate the effect of glucose on tac-promoter activity, we added glucose to the culture media. According to Figure 2 and Table 1, expression of the antigenic fragments of hyaluronidase A in NB 1.5x without glucose had the highest protein expression levels compared with NB 1.5x with glucose, LB broth with glucose, and LB broth without glucose. The results showed that although in lower glucose concentrations the tac-promoter activity will be increased, it will be followed by a reduced rate of protein production.
4.2.2. Comparison of Temperature Induction
Another important factor affecting protein expression was temperature shift. Hence, protein expression levels were determined at 30°C and 37°C. The results suggest that the expression level of this recombinant protein in 37°C was higher than that in 30°C, while the solubility of the expressed protein was equal in both temperatures, as shown in Figure 2. This condition was used in all the induction experiments.
4.2.3. Comparison of the Incubation Time on HylA Production
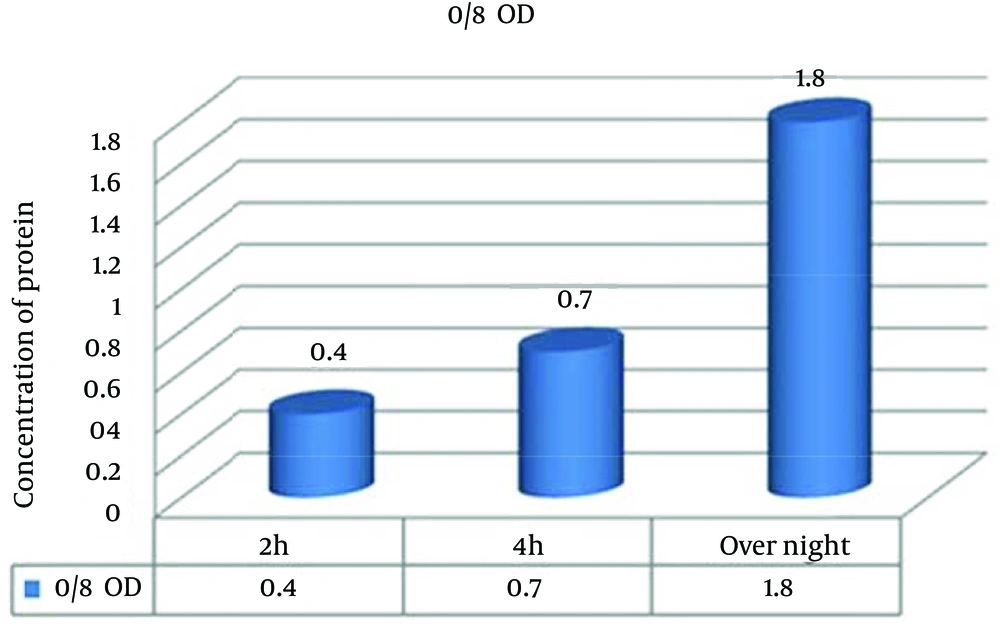
To analyze the role of incubation time on protein expression, the samples were incubated at three different times. The data showed that the pick of expression level of antigenic fragments increased in 24 hours after induction in NB 1.5x without glucose (Figure 3).
4.2.4. Effect of Isopropyl β-D-1-Thiogalactopyranoside Concentration on HylA Production
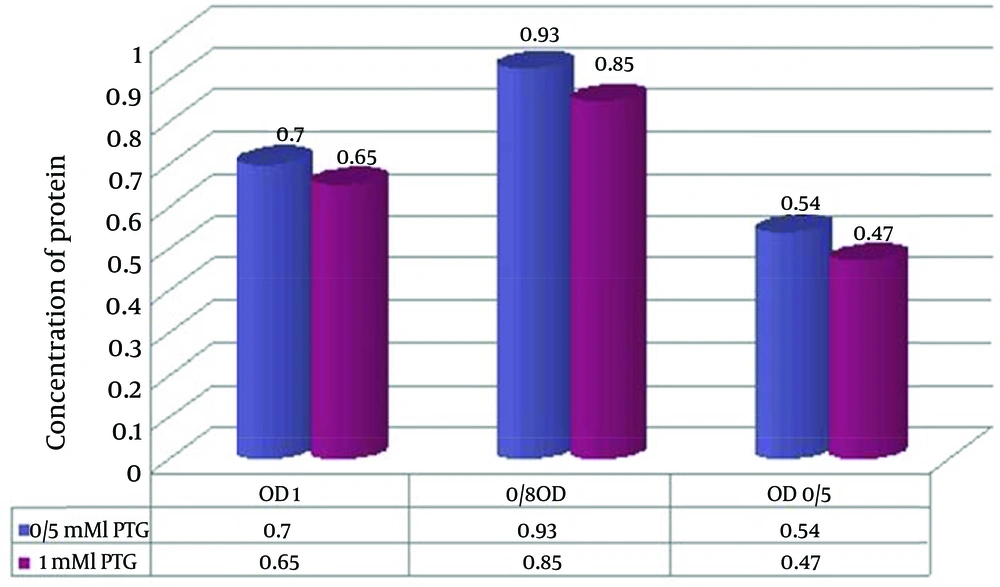
Another parameter that affected the protein expression was IPTG concentration. Protein expression level in 0.5 mM IPTG was more effective than in 1 mM. Trends were determined using column regression analysis (Figure 4).
4.2.5. Effect of Different Optical Densities on HylA Production
As showed in Figure 3, the middle column has OD = 0.8 which is the optimum for enzyme expression. ODs of 0.6 and 1 are in two other columns in which the expression of the enzyme is reduced. A higher level of HylA concentration was found in OD = 0.8.
4.3. Biological Assay
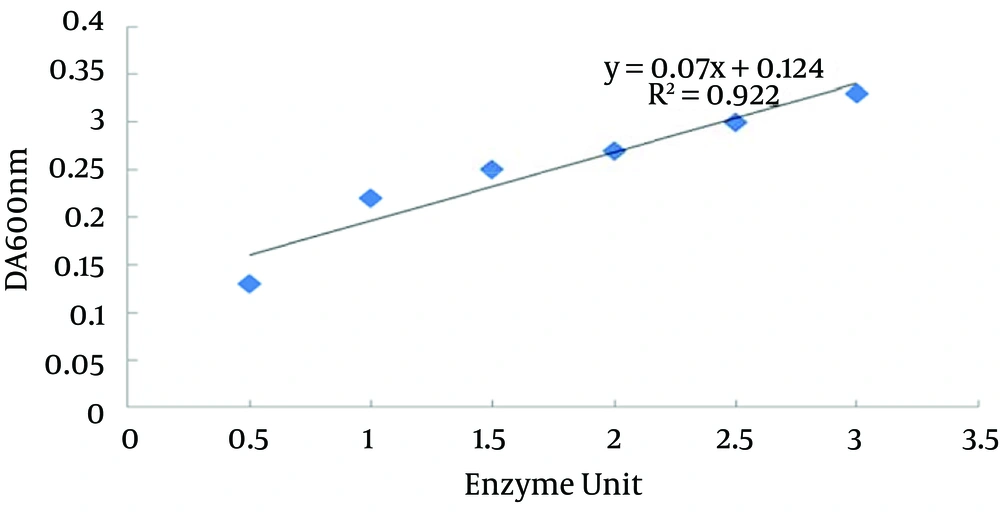
To determine the enzymatic activity, recombinant protein turbid metric assay was performed. According to the standard curve from serial dilutions of a commercial hyaluronidase stock, the results revealed that the concentration of produced recombinant protein was about 0.77 U/mL (Figure 5).
5. Discussion
E. coli is well studied and its expression system is necessary for characterization of proteins. The maximum protein expression of bacteria is important for industrial purposes. There are many adjusting factors in the recombinant protein expression system of E. coli (31). Mello and colleagues applied esurience-related promoter to adjust the heterologous gene expression, once the culture got to the stationary phase (32). Galloway et al. and Chae et al. showed their efforts in inducing protein overexpression at late log or stationary phase (33, 34). Mahmoudi et al. performed initial experiments on chemical and temperature-inducible expression systems to identify appropriate expression conditions to improve the production of recombinant streptokinase. They showed that the amount of recombinant streptokinase increased in the medium containing 2.4% glucose, 480 μg/mL tryptone, 580 μg/mL MgSO4 in 37°C (35). Jiang et al. explained the stationary phases of three E. coli systems, BL21 DE3 (pET), DH5a (pGEX) induced with lactose, and TG1 (pBV220) induced with heat shock, which could overexpress diversified genes, including three whose products are deleterious to the host cells. Their results offered a better strategy for recombinant protein preparation in E. coli (36).
In this study, we showed that an important advance in HylA yield can be obtained by changing the expression conditions. The culture medium ingredients have significant effects on the protein expression ability of this enzyme. Our investigations showed that the gene encoding for antigenic fragments of hyaluronidase has better protein expression ability in NB 1.5x without glucose. The presence of glucose leads to agglomeration of acetate, which is a cell growth inhibitor (38). In addition, lac operon in pGEX expression system is sensitive to glucose effects. When glucose is present, the genes for lactose metabolism are transcribed to a small magnitude. Furthermore, maximal transcription of the lac operon happens only when glucose is absent and lactose is present. The action of cyclic adenosine monophosphate (cAMP) and a catabolite activator protein produce this consequence. When glucose is absent, cAMP binds to the catabolite activator protein and transcription of the lac promoter will be increased by increment binding of T7 RNA polymerase to the lac promoter (37).
In the present study, we used E. coli BL21 DE3 which has cytoplasmic protease genes such as lon, ompT, degP and htpR. These genes contribute to overexpression of the recombinant protein (38). Our result showed that temperature had direct effects on enzyme expression. Higher temperature causes higher level of enzyme expression which might be due to increase in correct folding of target proteins. OD is another effective factor on the expression of enzyme. The tac operon induction after the stationary has been a better strategy for plasmid stability and preparation of recombinant protein synthesis system (33).
Finally, we found that IPTG concentration is the most effective variable, acting as trigger for gene transcription in lac operon. IPTG stimulates the beta-galactosidase activity. Addition of IPTG to the medium causes induction of T7 RNA polymerase activity for DNA transcription for production of a recombinant plasmid. More than 0.5 mM IPTG causes cell lysis of E. coli BL21 DE3 which effects gene expression and formation of inclusion body (33). The commercial availability of hyaluronidase A has improved the diagnosis of S. pyogenes infection. Assessment of hyaluronidase activity is necessary due to its applications in diagnosis of diseases. Since the recombinant protein of the antigenic fragments of this enzyme has similar antigenic property and enzymatic activity, it can be used for diagnosis of S. pyogenes infection. Therefore, the necessity of new purified and effective recombinant hyaluronidase antigenic fragments is not only of academic importance but also of practical interest (29, 39).
The recombinant hyaluronidase can be assayed by spectrophotometric methods which detect the formation of an unsaturated bond during the catalysis of this enzyme. Stern et al. have engendered a novel ELISA-like rapid and easy assay for hyaluronidase. This method is based on a high affinity biotinylated HA-binding peptide derived from tryptic digests of proteoglycan core protein of bovine nasal cartilage and the avidin-biotin reaction. In this method, a standard curve was plotted for hyaluronidase activity, to which all unknown enzyme samples were compared (21). The colorimetric Morgan-Elson assay method is an insensitive method based on a reducing N-acetyl-D-glucosamine terminus (24). Takahashi et al. expressed a fluorimetric Morgan-Elson assay method for hyaluronidase activity. This method has high sensitivity, is rapid, and has a detection limit of 5x 10-3 NFU/mL of bovine testicular hyaluronidase after one hour incubation. This method is more useful for measurement of low hyaluronidase activity in biological samples such as human and rabbit serum hyaluronidase (22). Chen et al. has described a new micro-method for assessment of hyaluronidase activity. This method is based on modified disc gel electrophoresis and chondroitin sulfate is used as a substrate. This is a sensitive method for assessment of hyaluronidase activity in serum and urine (23). Pearce et al. has explained that turbidimetric method is based on the turbidity-reducing activity of hyaluronidase in 10 minutes of incubation. This method is easy and cost effective and the hyaluronidase activity of most bacteria can be demonstrated (40).
The result of turbid metric assay demonstrated that the concentration of producing recombinant protein was about 0.77 U/mL. The result of the present study indicated that this fragment had enzymatic activity; revealing the active site and antigenic dominance in the amino acid sequence of 838 to 2320 of this protein. On the other hand, the enzymatic activity of this recombinant protein was similar to commercial hyaluronidase. Hence, this fragment is a good candidate for producing large amounts of commercial recombinant hyaluronidase. In addition to antigenic properties, hyaluronidase antigenic fragments have an enzymatic activity similar to the whole gene. This matter can be used in molecular biology, particularly in further investigations, to obtain a small protein with both enzymatic and antigenic properties. Data indicated that the antigenic fragment of the recombinant hyaluronidase protein from S. pyogenes had considerable enzymatic activity and can be used for medical purposes.