1. Background
Human cutaneous infections caused by a homogeneous group of keratinophilic fungi called dermatophytes. These ubiquitous fungi are responsible for the most common fungal infections in major populations globally (1). Microsporum canis is the main agent of dermatophytosis in cats, its natural host and is responsible for a frequent zoophilic that has increased in several European countries (2, 3). M. canis is the major common cause of baldness in recent years in Iran. There also are reports of pseudomycetoma by M. canis. M. canis is a zoonosis fungus in dogs, cats, probably horses and monkeys that can lead to infection. While it is not geophilic but the isolates from soil cause ectothrix form of tinea capitis in human. In case of infection due to M. canis, hairs show greenish-yellow to brilliant fluorescence radiation if tested with Wood's lamp. This fungus also attacks to the skin with no hair (4, 5). Identify M. canis virulence factors have focused on proteases, including keratinases (6). This pathogenic dermatophyte is eukaryote and chemical treatment with antifungal drugs may also affect host tissue cells (7).
Many species of Actinomycetes, particularly those belonging to the genus Streptomyces, are well known as antifungal biocontrol agents that inhibit several plant pathogenic fungi (8, 9). The antagonistic activity of Streptomyces to fungal pathogens is usually related to the production of antifungal compounds (10, 11) and extracellular hydrolytic enzymes (11, 12). Chitinase and β- 1, 3-glucanase are considered to be important hydrolytic enzymes in the lysis of fungal cell walls, as for example, cell walls of Fusarium oxysporum, Sclerotiniaminor, and S. rolfsii (13). The antifungal potential of extracellular metabolites from Streptomyces against some fungi was previously reported (8, 10). However, data related to the antagonistic ability of the extracellular metabolites of Streptomyces strains to suppress the growth of the fungal pathogens Colletotrichum gloeosporioides and S. rolfsii having a broad host range are limited (9).
2. Objectives
This study aims to determine the effect of soil Actinomycetes antifungal activity against M. canis isolated from soil and identification like Streptomyces DNA using gene 16SrDNA.
3. Materials and Methods
3.1. Sampling, Isolation and Culture of Actinomycetes
To obtain Actinomycete isolates over 100 random soil samples from different areas of Kerman Province (Sangi Park, Sahebazzaman the mosque, Motahari Park and Neshat Park) from depth of 10-20 cm of the ground were taken, air dried and passed through 0.8 mm mesh. Tenfold serial dilutions prepared in water (10 - 4, 10 - 5 and 10 - 6) then cultured on Casein Glycerol Agar (CGA) (casein 0.3 g/L; KNO3 2.0 g/L; NaCl 2.0 g/L; MgSO4.7H2O 0.05 g/L; CaCO3 0.02 g/L; FeSO4.H2O 0.01 g/L; KH2PO4 2.0 g/L; and agar (Merck, Germany) 18.0 g/L) and incubated at 29°C for 5 - 7 days. From day five on, Actinomycete colonies were isolated in pure cultures on CGA. Full grown cultures kept refrigerated before use.
3.2. In Vitro Bioassays to Detect the Antifungal Activities
Bioassays were performed using Disk-method technique. In this method, six mm agar plugs of six-day old cultures of Actinomycetes on CGA medium were placed on lawn cultures of the pathogen on Potato Dextrose Agar (PDA) (Merck, Germany). Plates incubated at 28°C for 10 days. Actinomycetes with clear inhibition zones were recorded as positive and selected for further evaluations. Plain agar plugs were used as controls. All bioassays were performed in triplicates and the mean values calculated.
3.3. Determination of Minimum Inhibitory Concentration (MIC)
To determine the MIC, concentrations of crude sap of each antagonist was prepared as 10, 5, 2.5, 1.25, and 0.625 mg/mL in DMSO: MeOH (Merck, Germany) (1:1, v/v) with suspension of M. canis conidia and tested in well-method technique against the pathogen and incubated at 29°C for 12 days. The lowest concentration with growth inhibition was selected as MIC. All bioassays performed in triplicates and the mean values calculated.
3.4. Production of Extracellular Enzymes
Lipase activity test: Growth media containing 10 g peptone (Merck, Germany), 5 g NaCl and 15 g agar/L was prepared and autoclaved. After autoclaving, 10 mg of Tween 80 was added to the media and mixed gently. Disk plugs (6 mm) of six-day old cultures of antagonist were prepared and placed on the media as in disk-method bioassay. Hydrolysis of Tween 80 by the bacterium presented as distinctive zone around the bacterial plug (14).
3.5. Proteolytic Activity Test
Minimal medium containing one g of sucrose (Merck, Germany), three g of casein (Merck, Germany), 2 g of calcium chloride and 15 g of agar/L in distilled water was prepared and autoclaved. 6 mm antagonist disk plugs were prepared and placed on the media as in disk-method bioassay. Hydrolysis of casein and production of clear zones were evaluated positive (15).
3.6. Amylase Activity Test
To 5% water agar, 2% (w/w) starch was added, autoclaved and poured in Petri dishes. After cooling, 6 mm antagonist disk plugs were prepared and placed on the media as in disk-method bioassay. Hydrolysis of starch and production of clear zones were evaluated positive (16).
3.7. Chitinase Activity Test
Nutrient agar was supplemented with 4% (w/w) colloidal chitin (Sigma), autoclaved and poured in Petri dishes. After cooling, 6 mm antagonist disk plugs were prepared and placed on the media as in disk-method bioassay. Hydrolysis of colloidal chitin and production of clear zones were evaluated positive. In this media, chitin was used as the sole carbon source and, therefore, strains with chitinase activity, hydrolysed chitin and transparent halo around their colonies were evaluated positive and Electron microscope studies of active isolates were performed according to the relevant protocol (17).
3.8. Genetic Analysis of PCR
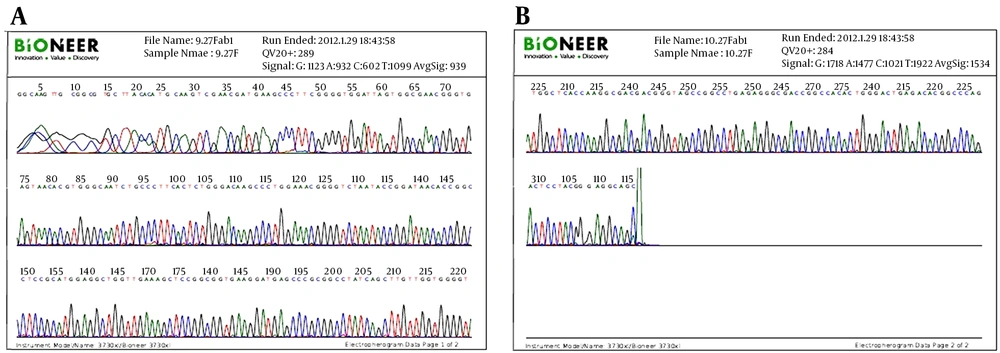
Extraction of bacterial DNA: From 5 day old bacterial culture, 1.5 mL transferred to sterile microtube and centrifuged for 5 minutes at 7500 g till bacteria precipitated. The supernatant discarded, the pellet received 100 μL protease buffer and kept at 95°C for 10 minutes solution was added to sediment bacteria according to the kit of Bacterial DNA extraction Owned by Sina Colon Iran. The lysis solution was given a gentle shake for 10 minutes at 37°C. 100 μL of the sample with 400 μL lysis solution were mixed and shake for15 - 20 seconds to be rotated. Then 300 μL of alcohol was added to microtube for 20 minutes at -20°C. The microtube was centrifuged for 10 minutes at 12000 g. The supernatant was discarded and the microtube gently was stroke on a paper for 2-3 seconds to remove the remaining precipitated liquid. One mL of wash buffer added to microtube for 3 - 5 seconds and slowly moved into rotator. It was then centrifuged at 12000 g for 5 minutes and the supernatant was removed. The microtube was kept to dry for 5 minutes at 65°C. After PCR, 45 μL of the PCR products including of the forward primer and molecular identification based on 16SrDNA was sent to the Bioneer Company for sequencing (18). Molecular identification: Sequences were identified using BLAST from National center for Biotechnology Gene Sequences in the database (http:www.ncbi.nlm.gov/BLAST/). This molecular method for identification of sequenced isolates using the 16SrDNA was found at the species level.
4. Results
4.1. Actinomycetes Isolated From Soil
From 10-4 - 10-6 dilutions of soil samples, over 100 Actinomycetes isolated in pure cultures on which further investigations performed. In vitro antifungal activity of Actinomycete isolates: Bioassay result of Actinomycete isolates Ks8 and L1 against the tested dermatophyte evaluated in disk-method technique. The inhibition zones in which no visible growth of the pathogen observed are representative of antifungal activity.
4.1.1. Determination of Minimum Inhibitory Concentration (MIC)
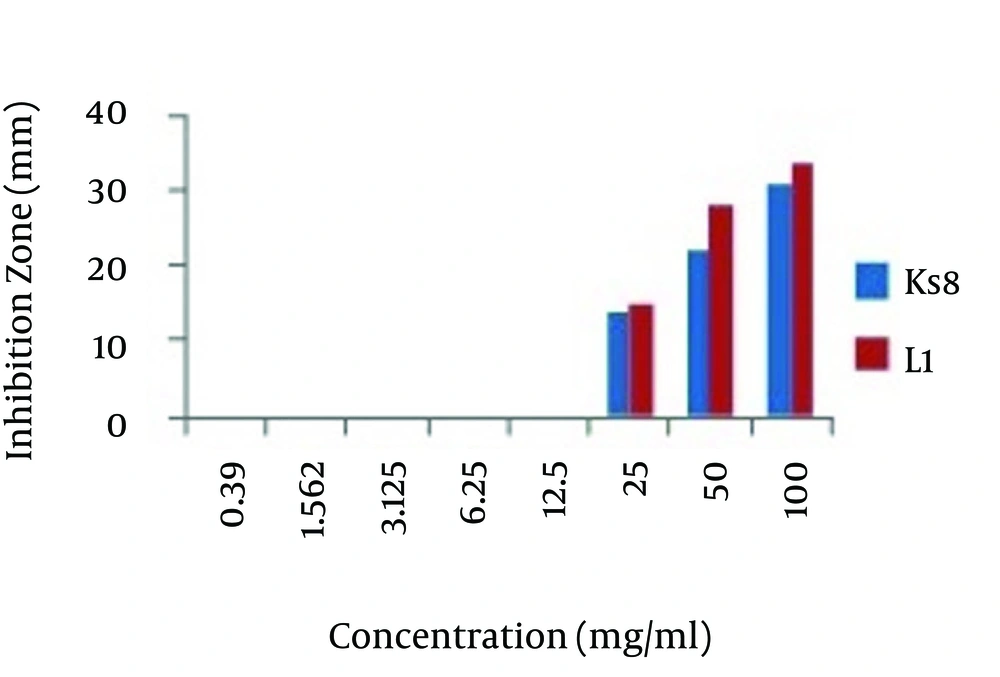
As shown in the Figure 1, The MIC for both Actinomycete isolates of L1 and Ks8 isolates determined as 25 mg/mL.
4.2. Enzymatic Bioassays
4.2.1. Lipase Activity
L1 isolate had varying degrees of lipase activity and could hydrolyze tweens. Sedimentary halo formation around colonies was produced by the L1 isolate of Actinomycete.
4.2.2. Proteolytic Activity
Extracellular protease activity of Ks8 and L1 Actinomycete isolates was indicated by their ability to hydrolyze casein (production of clear halo around colonies).
4.2.3. Amylase Activity
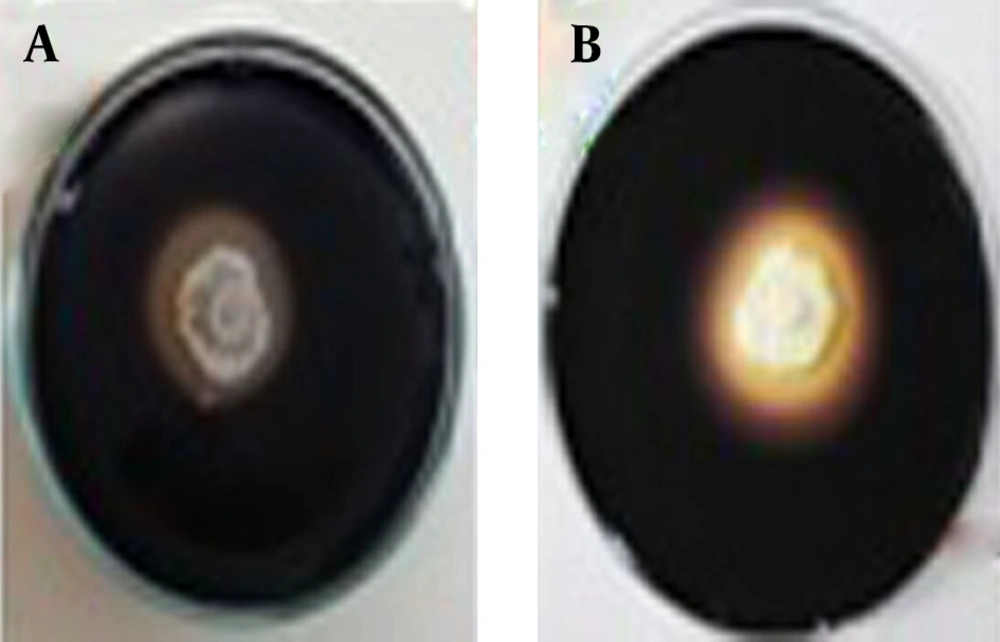
L1 and Ks8 isolates was able to break down the starch polymer and created the colorless zone around the colonies after adding the substrate as shown in the Figure 2.
4.2.4. Chitinase Activity
L1 and Ks8 isolates had the best result for chitinase activity. It showed a halo around the colony after growing on the chitin media.
4.2.5. Electron Microscope Studies
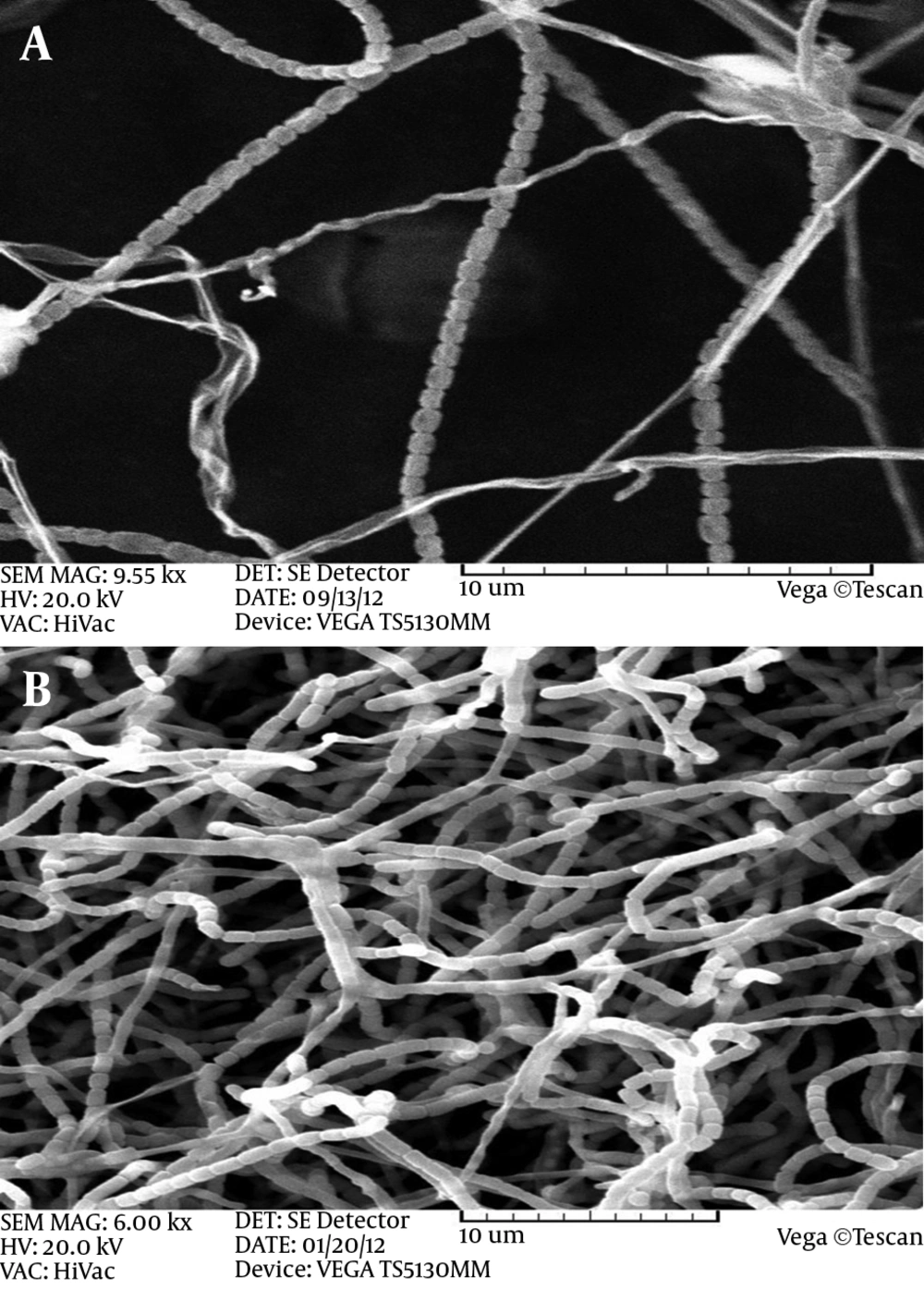
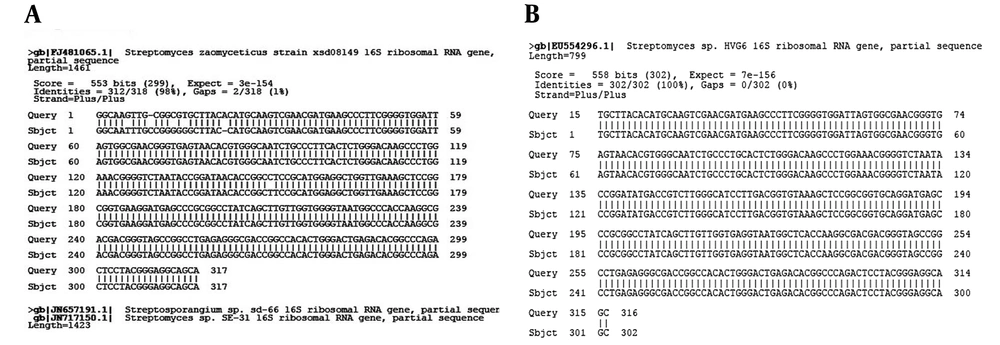
Electro micrographs of both Actinomycete isolates of Ks8 and L1 indicated that both isolates bear long spore chains in which spores had smooth surfaces. Figure 3 shows the spore chains and mycelial morphology of both isolates. Phylogenetic analysis of the nucleotide sequence of the 16SrDNA gene of active Actinomycete isolates: 16SrDNA sequences using specific primers was amplified by PCR technique (Figures 4 and 5). Comparison of 16SrDNA sequences active Actinomycete isolates with sequences available in Gen Bank/DDBJ/EMBL using Blast. Nucleotide analysis showed that isolate Ks8 had maximum homology (98%) to Streptomyces zaomyceticus strain xsd08149 and L1 displayed 100% homology to Streptomyces sp. HVG6 (Figure 6).
5. Discussion
It has been demonstrated that 85 percent of antibiotics which produced and used from Actinomycetes are mainly from various species of Streptomyces (19). In this study, we tried to screen and evaluate Actinomycetes isolated from the soil of Kerman Province for their antagonist activity against M. canis the causative agent of human dermatophytosis. Over 100 isolate of Actinomycetes cultures were isolated from different areas of Kerman’s soil and then the in vitro tests were performed. Actinomycetes were isolated when their morphological features and their pigmentation revealed after seven days (20). Lakshmipathy and Kannabiran (2009) introduced 100 isolates of Actinomycetes isolated from soil (21). In an initial screening to find appropriate antifungal effect of isolates against M. canis, biological screenings performed by agar disk method. The isolates of L1, D5, Ks10, Km2, Kn10, Ks8 and Ks1 showed highest antagonist activity against M. canis based on production of zones of inhibition. In a similar study by Shahidi Bonjar, et al. (2006) they identified twelve strains out of 130 isolates of Actinomycetes against Phytophthoradrexler (22).
In another study in Bangkok (2008) similar to what we did; 10 Actinomycetes isolated from 146 soil samples showed antifungal effect against the three pathogenic fungi (23). Augustine and colleagues (2005) also conducted a similar study that proved Streptomycesrocky AK39 has anti-dermatophyte effect (24). Based on their MIC studies they demonstrated only three isolates from more than one hundred strains showed to have antifungal compounds against the tested fungi. In this research antifungal activity of the isolated strains was demonstrated and it showed the importance and potential for further investigation. Lateral diffusion is a physicochemical approach in which microorganisms using it as indicators of active compounds (25). The study conducted by Zakir and colleagues (2002) developed a MIC between 32 - 64 mg/mL for the active metabolite isolated from Streptomyces species against four Gram positive and Gram-negative bacteria respectively (26). As isolates evaluated in this study able to control fungi at a very low concentration then it can be concluded that the antagonistic effects are well studied in the control of microorganisms.
The SEM images obtained from isolates of Actinomycetes showed some form spores, mycelia and spore chains as their morphology revealed. Research in order to identify and classify different species of Streptomyces and electron-type levels of spores in Streptomyces species were reported straight. Also the form of spore chains using electron microscopic studies of Streptomyces species, and Streptomycesgriseus was a spiral, circular and flexible straight up (27). Streptomyces lytic activities are mainly the result of lyse enzymes such as chitinase and gluconase (28). Chitin is a major component of the fungal cell wall and as substrate for chitinase enzyme (29).
The inhibition of fungi by Streptomyces may be related to the production of chitinase (30). In a study by Baharlouei, et al. (2009) they reported 18 Actinomycete strains out of 110 isolates had strong chitinase activity (31). Since the fungal cell wall contains chitin fibers and the matrix of proteins proteases play a significant role in the degradation of the wall (32). The role of extracellular proteases in various biocontrol processes also was shown in pathogenic fungus Trichoderma harzianum (33). In this research we demonstrated that Streptomycetes antagonists had antifungal effects over M. canis. The outcome of this study can be used for making new antifungal drugs in the future. In this case many various elements should be studied such as evaluation on animal's models for short and long term side effects, carcinogenic and teratogenic effects and environmental impacts. The topical evaluations on volunteers also should be considered before any real conclusion.