1. Background
Escherichia coli isolates of the Enterobacteriaceae family are important causes of infections, especially urinary tract infections (UTIs) (1). It is estimated that about 35% of healthy individuals suffer from symptoms of UTI at some stages in their lives (2). Since 1980 the beta-lactam antibiotics have been widely used for the treatment of serious infections which were made by Gram-negative bacteria, but resistance against these antibiotic groups was developed quickly worldwide (3, 4). In general, extended-spectrum beta-lactamase (ESBL) -productions are most frequently observed in E. coli isolates and rarely observed among other bacteria (3). The most important mechanism of resistance against beta-lactam antibiotics is beta-lactamase production (5).
The ESBLs which are enable to hydrolyze and inactivate a wide variety of beta-lactams including third generation cephalosporins, penicillins, and aztreonam (6) are plasmid mediated enzymes. Most of ESBL enzymes that have been observed in many countries (8) are members of TEM and SHV families (7). In Gram-negative bacteria, TEM-1 is the most commonly encountered beta-lactamase. Up to 90% of the ampicillin resistance in E. coli is due to TEM-1 production (8). Although TEM-type beta-lactamases are most often found in E. coli and Klibsella pneumoniae, they are found in other species of Gram-negative bacteria with increasing frequency (8).
Bacterial resistance to antimicrobial is a global problem; thus; discovery of newer antibacterial agents is always necessary. As a result of this problem, researchers are usually focusing on natural products to develop better medications against multidrug resistant microbial strains (9). A wide variety of secondary metabolites, which are used either directly as precursors or as compounds in the pharmaceutical industry are produced by plants. It is expected that other than plant used by antibiotics, plant extracts showing target sites will be more active against drug-resistant microbial pathogens (10). The antimicrobial potency of plants is believed to be due to tannins, sponins, phenolic compounds, essential oil, and flavonoids (11).
Myrtus communis L. (Myriaceae) is a perennial shrub belonging to the Myrtaceae family and subfamily (Myrtoideae). The leaves contain tannins, flavonoids such as quercetin, catechin, and myricetin derivatives along with volatile oils (12). The antimicrobial activity of essential oil of leaves (M. communis) against E. coli was recorded in some studies (13, 14). Cumin (Cuminum cyminum L) is an aromatic plant of the Apiaceae family. The antimicrobial characteristics of herbs are due to various chemical compounds including volatile oil, alkaloids, tannins and lipids that are presented in their tissue (15). Cuminum cyminum L. essential oil exhibited stronger antimicrobial activity against E. coli (16). In the study of Soniya, the largest diameter of an inhibition zone was observed from methanol extracts of C. cyminum against E. coli (17). Marrubium vulgare L as a medicinal plant possesses tonic, aromatic, stimulant, expectorant and diuretic properties. It was formerly much esteemed in various uterine, visceral and hepatic affections and in phthisis (18).
Some biological and antibacterial properties of essential oil extracted from this plant and their constituents have been recently investigated. The study of Kahlouche-Riachi revealed that the M. vulgare L. methanolic extract showed higher antibacterial activity on E. coli with the minimum inhibitory concentration (MIC) of 0.195 mg/mL (19). In the study of Bokaeian, the least MIC value of extract M. vulgare against Staphylococcus aureus was 2.5 mg/mL and the highest MIC value of essential oil M. vulgare was 2.5 mg/mL (20). Peganum harmala has been known as “Espand” in Iran. It belongs to Zygophyllaceae family, Zygophyllales contains about 22 genera and more than 250 species (21). Darabpour showed the MIC of 0.625 mg/mL against E. coli isolates (22). In another study, extract of the seeds of P. harmala at concentration of 0.3 mg/mL had a greater inhibitory effect on E. coli O157 (23). The results from the study of Shiri showed that P. harmal and M. communis have a potent antimicrobial activity against Gram-positive (S. aureus) and Gram-negative (E. coli and E. cloacae) bacteria, respectively (24). In the Amaranthaceae family, Amaranthus retroflexus is a species of flowering plant that is known by these common names, including red-root amaranth, red-root pigweed, ped-rooted pigweed, common amaranth, and common tumbleweed (25).
2. Objectives
This study aimed to investigate the antibacterial activity of some plant extracts against extended-spectrum beta- lactamase- producing E. coli isolates harboring the TEM gene.
3. Materials and Methods
3.1. Isolation of Escherichia coli
The urine culture of the patients suffering from urinary tract infections hospitalized in Boo-Ali Hospital (Zahedan, south-eastern Iran) were collected from September 2010 to March 2011. First the samples were examined by microscopic Gram stain examination. Samples with Gram-negative results were inoculated on plates of nutrient agar, Clede agar, MacConkey's, and blood agar (Merck, Germany) and then incubated at 37°C for 24 hours. The colony that showed fermenting of lactose on MacConkey agar and Cled agar media were purified and identified according to their morphology as circular, rose-pink to red colonies on MacConkey agar medium and yellow colonies on Cled agar. The isolates were identified by some biochemical reactions (e.g. catalase enzyme, potassium hydroxide test, Indole and methyl red test, Voges Proskaur reaction, urease and citrate, H2S and oxidase test). A total of 120 strains of E. coli were isolated according to the following steps.
3.2. Extended-Spectrum Beta-Lactamase Detection by Confirmatory Tests
3.2.1. Double Disk Synergy Test
A disk of ceftriaxone (30 μg) and ceftazidime (30 μg) (Patan Teb-Iran) were placed 16 to 20 mm apart from the Augmentin disc (center to center). After incubation (37°C for 24 hours), the zone of cephalosporin disc towards the clavulanic acid disc was considered as the ESBL producer (26).
3.2.2. Phenotypic Disc Confirmatory Test
The test was performed as recommended by CLSI (Clinical and Laboratory Standards Institute). Disks of ceftazidime (CA) 30 μg and ceftazidime-clavulanic acid (CAC) 20 + 10 μg or ceftriaxone (CE) 30 μg and ceftriaxone clavulanic acid (CEC) 20 + 10 μg were placed on MHA (Muller Hinton Agar) at a distance of 30 mm between each other. An increase in the zone diameter (= 5 mm) for CAC versus CA or CEC versus CE is confirmed as ESBL-producing organisms (26). E. coli ATTCC 25922 was used as a control strain.
3.3. DNA Extraction and Polymerase chain Reaction
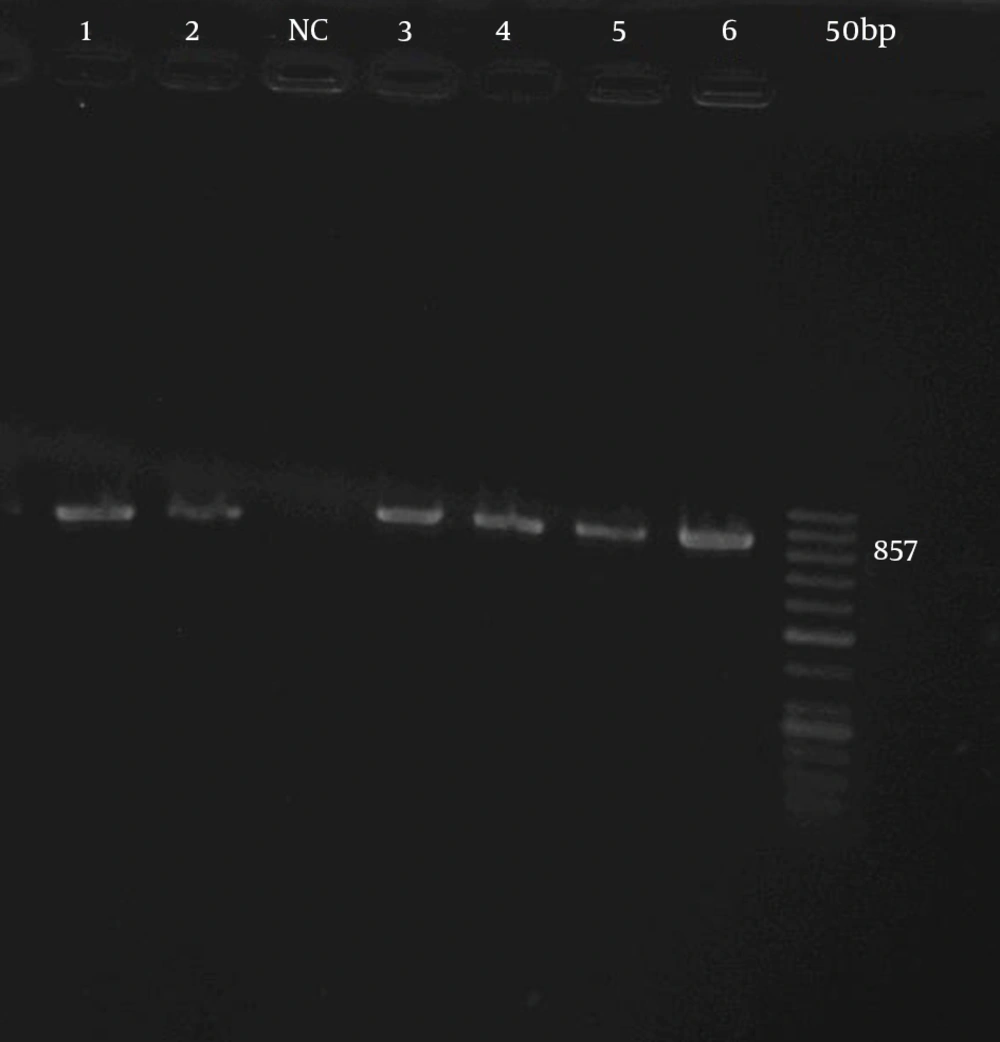
The colonies of ESBL-producing organisms were suspended in (Tris + EDTA [Ethylene Diamine Tetra Acetic acid). The buffer and their DNA were extracted through simple boiling. As described previously, the PCR method for TEM gene detection was performed with minor modifications (11). Briefly, specific primers for the genes (forward primer 5´-GAGTATTCAACATTTCCGTGTC -3´; reverse primer 5´-TAATCAGTGAGGCACCTATCTC - 3´ for TEM gene) (Gene Gostar- Iran) were used for PCR amplification that resulted in 857 bp PCR products for TEM gene. The PCR mixture consisted of 10 pmol of each primers, 1 μL DNA sample (3 μg/μL), 1.5 mM MgCl2, 0.2 mM each dNTP, and 5 u Taq DNA polymerase (CinnaGen Co, Iran) in a total number of 50 μL of the PCR reaction. Amplification of the TEM gene was performed by the following program: initial denaturation at 94°C for 120 seconds and 35 cycles of 60 seconds at 94°C, 30 seconds at 52°C and 60 seconds at 72°C. 300 seconds at 72°C was considered for the final extension. Then, PCR products were analyzed by the agarose gel electrophoresis.
3.4. Determination of Minimum Inhibitory Concentration of Antibiotic
The MIC of antibiotics was defined using the bacterial broth dilution method used by Baron and Finegold (27).To evaluate the effect of antibiotics, the nutrient broth (Merck, Darmstadt, Germany) (pH = 6.5) containing different concentrations (512, 256, 128, 64, 32, and 2 µg/mL) were prepared from some antibiotics (ceftazidime, ceftriaxon, amikacin, gentamicin, ciprofloxacin; Farabi Pharmaceutical Co, Isfahan, Iran). As the control media, nutrient broth without antibiotic was used. The MIC was defined as the lowest drug concentration, which prevented visible growth of bacteria and E. coli ATTCC 25922 was used as control strain.
3.5. Plant Materials
The leaves of M. communis L (Myrtaceae), A. retraflexus (Amaranthaceae), M. vulgare (Laminaceae), and seed of P. harmala (Zygrophyllaceae), and C. cuminum L (Apiaceae) were collected in Iran (Zahedan and Kerman, south-eastern, Iran). Samples were identified and approved by a botanist at Kerman branch, Islamic Azad University's Herbarium and were dried at room temperature. Samples were crashed and transferred into glass containers and were preserved for the extraction procedures, which were performed in the laboratory.
3.6. Preparation of Extracts
As reported by Hanafy and Hatem, plants were properly dried and pulverized into a coarse powder (28). Each of 20 g grinded powders was soaked in 60 mL of ethanol (95%), separately for one day (shaken occasionally with a shaker). After one day of the dissolving process, materials were filtered (Whatman no. 1 filter paper).Then the filtrates were evaporated using a rotary evaporator. At last, 0.97 g of dried extracts were obtained and then stored at 4°C in air tight screw-cap tube.
3.7. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of Plant Extracts
The broth microdilution method was used to determine MIC and minimum bactericidal concentration (MBC) according to Yu et al. (29). All tests were performed in Mueller Hinton broth supplemented with Tween 80 at a final concentration of 0.5% (v/v). Briefly, serial doubling dilutions of the extract were prepared in a 96-well microtiter plate ranged from 0.3 mg/mL to 10.00 mg/mL (30). To each well, 10 μL of indicator solution (prepared by dissolving a 10-mg extract in 2 mL of DMSO) and 10 μL of Mueller Hinton Broth were added. Finally, 10 μL of bacterial suspension (106 CFU/mL) was added to each well to achieve a concentration of 104 CFU/mL. The plates were wrapped loosely with cling film to ensure that the bacteria did not get dehydrated. The plates were prepared in triplicates, and then they were placed in an incubator at 37°C for 18 - 24 hours. The color change was then assessed visually. The lowest concentration at which the color change occurred was taken as the MIC value. The average of 3 values was calculated providing the MIC values for the tested extract. The MIC is defined as the lowest concentration of the extract at which the microorganism does not demonstrate the visible growth. The microorganism growth was indicated by turbidity.
3.8. Sample Size and Statistical Analysis
Regarding the theoretical proportion, P1 = 0.6 and the sample proportion, P2 = 0.7 with α = 0.05 and β = 80% (according to the following formula) the calculated sample size was estimated. Therefore, approximately 120 samples of E. coli were examined.

Absolute and relative frequencies of antibiotics and plant extracts used against ESBL-producing E. coli isolates were calculated according to the MIC.
4. Results
In this study, 80 (66.6%) out of 120 E. coli isolates were ESBL-producing organisms. In PCR method the distribution of TEM gene in isolated ESBL-producing organisms were 50 (41.6%) (Figure 1). Among 50 ESBL-producing E. coli isolates, 20 had MICs of 128 µg/mL for ceftazidime and one of the isolates revealed the MIC of 8 µg/mL for gentamicin and ciprofloxacin. Fifteen isolates showed MIC of 128 µg/mL for ciprofloxacin and gentamicin. A total of 49 isolates were resistant to ciprofloxacin (MIC ≥ 8 µg/mL) (27) and 46 isolates were resistant to gentamicin (MIC ≥ 8 µg/mL) (27). Therefore, ciprofloxacin showed the least effect on the isolated E. coli. Twelve E. coli isolates showed MIC of 32 µg/mL for amikacin and overall 27 isolates were resistant to amikacin (MIC ≥ 32 µg/mL) (27). Hence, amikacin was the most effective antibacterial agent against E. coli isolates. The most frequent number of isolates (n = 19) demonstrated MIC of 256 µg/mL for ceftriaxon.
The results of MIC for different antibiotics were shown in Table 1. Different inhibitory effects of alcoholic extract from different plant extracts against most E. coli isolates are demonstrated in Table 2. The results in Table 2 showed that the ethanol extract of different plants had an inhibitory effect against most isolates. The most frequent number of ESBL-producing E. coli isolates (33 out of 50) had MIC of 5 mg/mL to ethanol extract of M. vulgare. Thirty-two out of 50 E. coli isolates had MIC of 2.5 mg/mL for ethanol extract of P. harmala. Therefore, P. harmala was the most effective among all the other studied plants. One E. coli isolate showed MIC of 0.62 mg/mL for the ethanol extract of A. retraflexus.
| Antibiotic | 2 µg/mL | 4 µg/mL | 8 µg/mL | 16 µg/mL | 32 µg/mL | 64 µg/mL | 128 µg/mL | 256 µg/mL | 512 µg/mL |
|---|---|---|---|---|---|---|---|---|---|
| AN | 5 (10) | 7 (14) | 6 (12) | 5 (10) | 12 (24) | 8 (16) | 7 (14) | 0 | 0 |
| Gm | 2 (4) | 2 (4) | 1 (2) | 2 (4) | 7 (14) | 9 (18) | 15 (30) | 10 (20) | 2 (4) |
| CAZ | 0 | 0 | 0 | 0 | 11 (22) | 7 (14) | 20 (40) | 12 (24) | 0 |
| CRO | 0 | 0 | 0 | 0 | 0 | 11 (22) | 13 (26) | 19 (38) | 7 (14) |
| Cip | 0 | 1 (2) | 1 (2) | 2 (4) | 3 (6) | 12 (24) | 15 (30) | 11 (22) | 5 (10) |
aAbbreviations: AN, Amikacin; Gm, Gentamicin; CAZ, Ceftazidime, CRO, Ceftraxon and Cip, Ciprofloxacin.
bData are presented as No. (%).
| Extract Plant | 0.3 mg/mL | 0.62 mg/mL | 1.25 mg/mL | 2.5 mg/mL | 5 mg/mL | 10 mg/mL |
|---|---|---|---|---|---|---|
| MSL | 0 | 0 | 1 (2) | 10 (20) | 30 (60) | 8 (16) |
| CCL | 0 | 0 | 2 (4) | 12 (24) | 12 (24) | 6 (12) |
| MV | 0 | 0 | 5 (10) | 5 (10) | 33 (66) | 7 (14) |
| PH | 0 | 0 | 4 (8) | 32 (64) | 14 (28) | 0 |
| AR | 0 | 1 (2) | 0 | 9 (18) | 28 (56) | 12 (24) |
aabbreviations: MSL, Myrtus communis L; CCL, Cyminum cuminum L; MV, Marrubiumvulgare; PH, Peganumharmala; AR, Amaranthusretraflexus.
bData are presented as No. (%).
5. Discussion
According to the results of this study, the prevalence of ESBL-producing E. coli was high, 66.6% by the disk diffusion test and 41.6% by TEM gene distribution using the PCR method. Most ESBLs have evolved by mutation from native β-lactamases, particularly TEM-1, TEM-2 and SHV-1. These parent enzymes are commonly found in Gram-negative bacteria, especially Enterobacteriaceae (31). TEM the most common of them are derivatives of TEM enzymes (32). The reported ESBL-producing rates in E. coli isolates from various parts of Iran varied from 8.9% to 67% (33); In Isfahan 51% of isolated E. coli was ESBL-producing bacteria (34). The results of this study showed the resistance to some E. coli isolates in MIC of 128 mg/L for amikacin and MIC of 512 mg/L for gentamicin, ceftriaxon and ciprofloxacin.
Historically, plants have been a good source of new drug compounds. Today, in many parts of the world, the extracts of medicinal plants are used for their antibacterial, antifungal, and antiviral properties (35). Some groups have reported that there is a relationship between the chemical compounds and the antimicrobial activity of the plants. In the present study, the ethanol extracts of M. communis, A. retraflexus, C. cuminum, M. vulgare, and especially P. harmala extracts had a potent antimicrobial activity against ESBL-producing E. coli. Peganum harmala has been used as a folklore medicine for the treatment of various conditions, such as lumbago, asthma, colic and jaundice (36). It has also been reported that this plant had antibacterial, antifungal and antiviral effects (37). The observed antibacterial activity of P. harmala might also be attributed to the high quantity of polyphenols, which are known to possess efficient antibacterial activity (22). It has been showed that seed and root extracts of P. harmala had the best antibacterial activity against some Gram-positive and Gram-negative bacterial species, especially E. coli (22, 38).
In the present study, the MIC of P. harmala ranged from 1.25 mg/mL to 5 mg/mL against the strains of E. coli and the most frequent number of isolates showing inhibitory effect were seen in the MIC of 2.5 mg/mL. M. communis were frequently used for treatment of burns, antidiuretics, antifungal, and anti- diabetic (39). The antimicrobial activity of essential oil of M. communis leaves against E. coli was reported (14). In our study the range of MIC was between 1.25 to 10 mg/mL and the most frequent number of isolates showing inhibitory effect was observed in MIC of 5 mg/mL. M. vulgare is helpful for bronchial asthma and nonproductive cough. It was formerly esteemed in various uterine, visceral and hepatic affections and in phthisis. The in vitro antibacterial activity of essential oil of M. vulgare's leaves showed a significant activity against microorganisms, especially Gram positive bacteria with inhibition zones and MIC values in the range of 6.6 - 25.2 mm and 1120-2600 μg/mL, respectively, whereas Gram-negative bacteria exhibited a higher resistance (40).
In our study the MIC of M. vulgare ranged from 1.25 mg/mL to10 mg/mL against the strains of E. coli and 33 strains demonstrated an MIC of 5 mg/mL. C. cuminum Seeds have cooling affect and had been used for gonorhoea, chronic diarrhea and dyspepsia. Externally they are applied in the form of poultice to allay pain and irritation of worms in the abdomen (41). Some researchers noted that cumin could be used as an emerging alternative antimicrobial agent for human applications. A study in Tehran revealed that cumin essential oils possessed antibacterial effect against all isolates of pseudomonas aeruginosa, with MIC and MBC value in the range of 0.015 to 0.25 mg/mL (42). In the present study the MIC of C. cuminum ranged from 1.25 to 10 mg/mL against the strains of E. coli, as 12 strains of E. coli showed MIC of 2.5 and 5 mg/mL.
The oil extracted from A. retraflexus contains mainly non-polar lipid compounds especially triglycerides. Amaranthus oil has a light to medium color, highly unsaturated liquid with a delicate, agreeable aroma and taste, allowing greater usage versatility. It also provides an excellent resource for omega series fatty acids (43). Results obtained from the study of Shiri showed that P. harmal and M. communis L have a potent antimicrobial activity against Gram-positive (S. aureus) and Gram-negative (E. coli and E. cloace) bacteria, respectively (30). In our study, the MIC of A. retraflexus ranged between 0.62 and 10 mg/mL against the strains of E. coli, as 28 strains of E. coli showed MIC of 5 mg/mL. Medicinal plants could be sources of compounds, which might be useful in managing beta-lactam resistant bacteria and ESBL-producing E. coli. However, further studies about the isolation of active compounds and the absence of toxicity of plant extracts are necessary to propose these plants as alternative approaches to resistance management.