1. Background
Cryptosporidium is one of the important genera belonging to the phylum Apicomplexa, which causes a widespread infection in humans and animals. The parasite is transmitted by food and water contaminated with infected human and animal feces, and causes cryptosporidiosis. Cryptosporidium hominis and C. parvum are the most reported species in human infections, while other species have also been reported (1, 2). Regularly, the infection is self-limited. However, in the case of malnutrition and immunocompromised individuals, it can cause life threatening disease. The incubation period is of almost one week, which is followed by diarrhea and other related gastrointestinal symptoms. The parasite completes sexual and asexual reproduction in the intestinal epithelial cells of a single host (3). Children and elderly are at risk for more severe infections (3, 4).
The diagnosis of Cryptosporidium infection is mostly based on the common microscopic methods. Therefore, because the sizes of the oocysts are similar to those of yeasts, the diagnosis becomes very difficult and the organism might be missed occasionally. Assays based on immunofluorescence methods are more sensitive than conventional staining methods (2, 5). Fecal Ag-capture enzyme linked immunosorbent assay (ELISA) is one of the most accurate diagnostic methods at present, and it is increasingly used in different studies, with good sensitivity and specificity (3, 6). We conducted a study to evaluate and compare the ability of Ag-detection ELISA and auramine phenol staining methods for diagnosis of Cryptosporidium infection in human stool specimens.
2. Objectives
The aim of the present study was to evaluate the antigen detection in stool specimens using ELISA and oocyst detection by auramine phenol (AP) staining methods, for the diagnosis of human cryptosporidiosis.
3. Materials and Methods
3.1. Sampling
In this study, 228 fecal samples from humans involved in farming activities, with mean age of 32.78 years old (min: 1 year, max: 79 years) were collected. The study subjects were livestock owners dwelling in rural areas of the outskirts of Hamadan City, West of Iran. The stool specimens were transported to the research laboratory, as soon as possible. Each stool sample was divided into two parts: one kept at -20˚C for Ag-capture ELISA and the other fixed in 10% formalin for AP staining. The demographic variables of the participants, including sex, age, direct or indirect contact with livestock, which livestock they were in contact, gastrointestinal symptoms and the place the livestock were kept, were collected by the questioners by interviewing the participants.
3.2. Concentration of Fecal Samples
Stool samples were concentrated using the formalin-ether sedimentation technique to achieve a better result in the AP staining method. The frozen samples were not concentrated for Ag-detection ELISA. The concentration of the samples was performed according to the following procedure: 1 gram of stool was emulsified by 7 cc of 10% formalin and passed through three layers of sterile gauze, then 3 mL ether was added and the mixture was shook vigorously, then centrifuged at 2000 rpm for 2 minutes and the supernatants were discarded. The sediments were used for the preparation of thin microscopic slides (7).
3.3. Staining Method
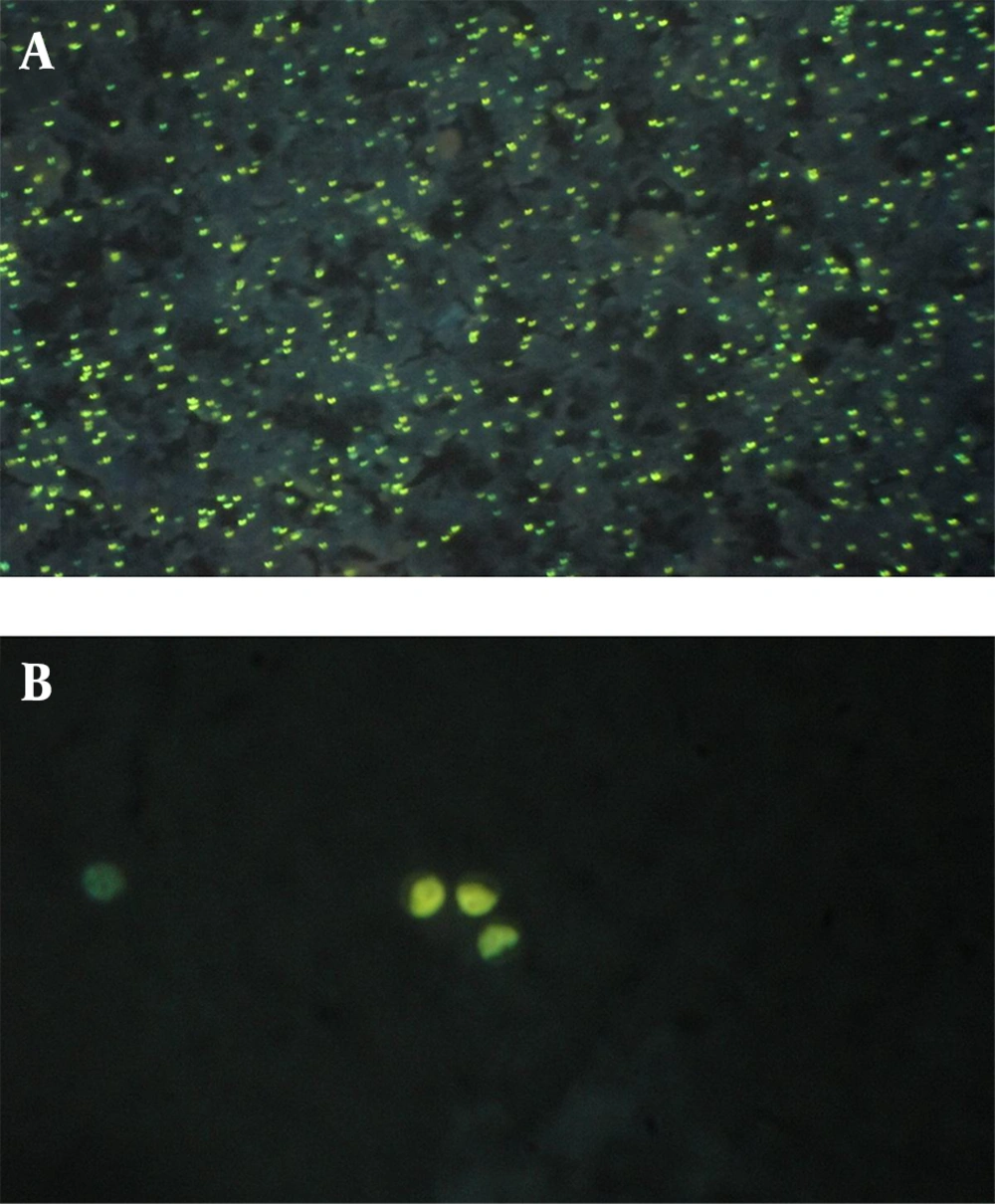
To perform the AP staining method, thin smears were prepared from concentrated materials, dried in room air and fixed with methanol, then rinsed in AP stain and washed in tap water to remove the excess color, then re-stained with potassium permanganate 1%, as counterstain. Then, the air dried slides were examined under a fluorescent microscope using UV filter with 450 nm emission (3). The oocysts of Cryptosporidium spp. were described as greenish yellow spherical objects under the florescent microscope (Figure 1).
3.4. Enzyme Linked Immunosorbent Assay
The qualitative Cryptosporidium assay was performed with Ag-capture ELISA kit (Cypress diagnostics, Langdorp, Brabant, Belgium). The ELISA procedure was performed according to the manufacturer’s manual. In brief, all ELISA reagents and frozen samples were brought to the room temperature, then diluted 1:11 with diluent solution and emulsified by shaking vigorously, and afterwards left stationary for about 30 minutes to precipitate. The ELISA test was started by adding 100 μL of supernatant, positive and negative controls into the wells. An amount of 100 μL of enzyme conjugate reagent was immediately added to the wells and the mixture incubated at room temperature for 60 minutes. The procedure continued by manually washing the wells five times with washing buffer reagent, using a multichannel microsampler. Then, 100 μL of chromogen/substrate reagent was added to each well and incubated in the dark room for 15 minutes. When the second incubation ended, 50 μL of stop solution were added to the wells and, in less than 15 minutes, photochromic measurement was performed at wave length of 450 nm, with the reference wavelength of ≥ 600 nm, using the ELISA reader device. The detection threshold was calculated according to the test manual of the company, by adding 0.15 to the absorbance of the negative control. The results with an absorbance superior to 10% of the threshold were considered as positive. The samples were considered negative when absorbance was 10% lower than the threshold. Also, the absorbance in between of the two cut-offs was considered as undetermined.
3.5. Data Analysis
Data were analyzed with the SPSS v.16 software (SPSS Inc., Chicago, ILL, USA) using McNemar and Chi-square tests.
4. Results
4.1. Auramine-phenol
Totally, 228 fecal samples were collected. Of these, three (1.3%) samples tested positive in the AP staining method (Table 1). Considering the demographic variables, the infection rate was significantly higher in diarrheic individuals (P = 0.048, OR: 5.769; 95% CI: 1.070 ‒ 31.112) and in participants who kept their livestock in their yard (P = 0.033, OR: 3.659; 95% CI: 1.569 ‒ 8.531). There was no significant relationship between the other demographic variables with the infection rate (Table 2).
aELISA, Enzyme Linked Immunosorbent Assay; AP, Auramine Phenol.
bAll of the Values are Presented as No.(%).
cELISA Used as Gold Standard Assumed Sensitivity and Specificity of 100%.
dAuramine Phenol Staining Method.
| Variables | Positive | Negative | Odds Ratio, OR | P value |
|---|---|---|---|---|
| Gender | 0.206 | |||
| Male | 3 | 132 | 1.705 | |
| Female | 0 | 93 | 1 | |
| In contact animal | 0.325 | |||
| Cattle | 0 | 71 | 1 | |
| Lamb/Goat | 3 | 154 | 1.461 | |
| Diarrhea | 0.048 | |||
| Yes | 1 | 13 | 5.769 | |
| No | 2 | 212 | 1 | |
| Livestock keeping place | 0.033 | |||
| Stall | 1 | 184 | 1 | |
| Yard | 2 | 41 | 3.659 | |
| Livestock Contact | 0.669 | |||
| Direct | 2 | 156 | 0.885 | |
| Indirect | 1 | 69 | 1 |
4.2. Cryptosporidium Fecal Ag-Detection ELISA
Eight (3.5%) out of 228 samples were positive for Cryptosporidium fecal Ag-detection using the ELISA method (Table 1). Considering the demographic variables, there was no significant relationship between the mentioned demographic variables and the infection rate (Table 3).
| Variables | Positive | Negative | Odds Ratio, OR | P value |
|---|---|---|---|---|
| Gender | 0.094 | |||
| Male | 7 | 128 | 1.504 | |
| Female | 1 | 92 | 1 | |
| In contact animal | 0.522 | |||
| Cattle | 2 | 69 | 1 | |
| Lamb/Goat | 6 | 151 | 0.093 | |
| Diarrhea | 0.403 | |||
| Yes | 1 | 13 | 2.115 | |
| No | 7 | 207 | 1 | |
| Livestock keeping place | 0.175 | |||
| Stall | 5 | 180 | 1 | |
| Yard | 3 | 40 | 2.062 | |
| Livestock Contact | 0.467 | |||
| Direct | 5 | 153 | 1 | |
| Indirect | 3 | 67 | 1.231 |
4.3. Comparison of the Results
The prevalence rate resulted from the ELISA was higher compared to that of the AP staining method. However, it was not significant statistically (P = 0.062) (Table 4). Because several researchers have expressed the idea that the performance of ELISA is similar to that of molecular methods (8), we assumed ELISA as gold standard, to calculate the sensitivity and specificity of the AP staining method. In this study, the sensitivity and specificity of the AP staining method were 37% and 100%, respectively.
| Variables | ELISA a | Total | P Value | |
|---|---|---|---|---|
| Positive | Negative | |||
| Auramine Phenol | 0.062 | |||
| Positive | 3 | 0 | 3 | |
| Negative | 5 | 220 | 225 | |
| Total | 8 | 220 | 228 | |
aAbbreviation: ELISA, Enzyme Linked Immunosorbent Assay.
5. Discussion
The very low prevalence rate for cryptosporidiosis among humans and the relatively high prevalence among livestock in Hamadan region has been described recently (9, 10). The present study showed a low prevalence in the similar population by fecal Ag-detection ELISA and the AP staining methods, which are suggested to be more sensitive diagnostic methods compared to the conventional, commonly used, modified Ziehl-Neelsen staining method. The results of the present study, and of several other researches, indicate that the ELISA method illustrates higher positive results than conventional staining methods, which is probably responsible to the reported superior accuracy of the method (8, 11-13). However, controversial results have been published as well (14, 15). Even the AP staining method itself, which was considered more sensitive than modified Ziehl-Neelsen staining method (3), showed less positives compared to ELISA. Cryptosporidium spp. oocysts were detected in only three samples with the AP staining method, while ELISA revealed eight positives.
As our results show, the ELISA has a good performance in low prevalence and, probably, in low oocyst excretion. The method is suitable for epidemiological studies, because it is rapid and easy to perform, while on the other side of the coin, it is not cost-effective. Although our study revealed the superior positivity of the ELISA method compared to AP staining, however, this was not significant (P = 0.062). Nevertheless, if we compare the two results, it becomes obvious that the higher prevalence observed in ELISA would show infection status probably close to its real prevalence in the society, in comparison to other less sensitive conventional methods.
Numbers of other similar studies, performed worldwide, are reviewed below. Rosenblatt et al. 1993 (12) evaluated the fecal Ag-detection ELISA and reported high sensitivity (93%) and specificity (99%) for it. They declare that ELISA is rapid, easy, and represents an accurate method for the diagnosis of Cryptosporidium infection. On the basis of their report, it can be concluded that staining methods, even highly sensitive microscopic methods, such as the AP staining method (3), cannot show the real epidemiological status of the infection. Our results showed the infection rate to be 3.5% (ELISA), which is higher than that observed by AP staining (1.3%). According to the results of the present study, the AP staining method possesses low sensitivity compared to the Ag-detection ELISA as a standard, and, if used for epidemiological studies, the results would not appear to be reliable.
Brook et al. 2008 (16) evaluated three tests, including modified Ziehl-Neelsen, AP staining and enzyme immunoassay methods for the diagnosis of Cryptosporidium infection. They express the idea that all the three methods are effective in diagnosing the infection in frozen and fresh cattle fecal specimens. In contrast, our findings illustrated a difference in the results of the two experimented methods, although the difference was not significant. Given the fact that our P value was close to valid statistical significance, a larger sample size would most probably reveal a significant difference.
In another similar study, Khurana et al. 2012 (14) evaluated modified Ziehl-Neelsen staining, AP staining, Ag-detection ELISA and molecular methods, for the diagnosis of cryptosporidiosis revealing sensitivities of 79.06%, 100%, 95.35% and 100%, respectively. In contrast to our results, in their study, the AP staining method showed higher positives than ELISA. They suggested that the AP method is a suitable method, because in their study it was highly sensitive and specific, simple, rapid and less costly or time consuming. However, our results show lower sensitivity for the AP staining method compared to Ag-detection ELISA as a golden standard in epidemiological studies with low prevalence status.
As far as authors’ knowledge, in the Iranian literature only one similar study has been performed by Dorostkar-Moghadam et al. 2004 (17) that evaluated the Enzyme Immunoassay (EIA) for the diagnosis of the Cryptosporidium infection compared to the modified Ziehl-Neelsen staining method confirmed by immunofluorescence assay. They concluded that, EIA is a sensitive, specific and simple method for the diagnosis of cryptosporidiosis. For epidemiological studies, especially on asymptomatic individuals from low-prevalence areas, Ag-detection ELISA may help to obtain an accurate prevalence rate or very close to statistical accuracy. Although it is easy to perform and rapid, but it is not cost-effective, especially in the case of large scaled studies.
Cryptosporidium infection rate is very low in Hamadan region (18) and, consequently, a larger sample size and increased budgets are needed to validate the results in larger studies. The insufficient funding for sampling and purchasing the ELISA kits was our main limitation in this study. For the epidemiological studies on the Cryptosporidium infection, especially on asymptomatic individuals in low prevalence regions, the Ag-detection ELISA method would reveal an accurate infection rate compared with conventional microscopic methods.