1. Background
BK virus (BKV) belongs to the human Polyomaviridae, initially isolated from urine sample of a 29-year-old male patient with renal blockage and failure at 1971. BK virus is an abbreviation of the name of the first patient whom the virus was isolated from. Usually, primary infection is occurred during childhood then the virus could be latent through life, especially in the kidneys and urinary system (1). About 60-90% of all adults have BKV antibodies in their circulation. It became reactive after immunocompromisation, status such as pregnancy or transplantation; in such patients BKV develops BKV-related renal failure and nephropathy. It is reported that BKV became reactivated in 10-60% of the cases of renal transplant patients from which 1-5% would undergo nephropathy; half of the patients with nephropathy reject their transplanted kidney. Risk factors of BKV-induced nephropathy are not well-known. However, immunosuppressant drugs, transplantation and BKV itself account as the major risk factors (2-5).
Isolated BKV from different locations of the world are grouped into four subtypes using serological and genotyping methods. Subtype I is the dominant one and has worldwide distribution; subtype IV is the more frequent after subtype I and mostly isolated from East Asia. Although subtypes II and III are isolated worldwide, their frequencies are low. Furthermore, according to the phylogenic investigations subtype I itself is divided into 4 subgroups including subgroups 1/a, 1/b-1, 1/b2 and 1/c; each one is distributed in a certain geographical location. Subtype IV, itself is divided into six subgroups in phylogenic studies including 4/a-1, 4/a-2, 4/b-1, 4/b-2, 4/c-1 and 4/c-2 (6, 7). According to previous reports, there are no clear correlation between BKV subtype determination and clinical outcome; thus, our study will have epidemiological benefits.
2. Objectives
According to our knowledge there are no data about BKV prevalence and its genotypes in southwest of Iran. Considering the high prevalence of renal failure and kidney transplant patients in this part and the role of BKV in graft rejection, this study aimed to determine the prevalence of BKV infection in renal transplant recipients referred to Golestan Hospital in Ahvaz, Iran. Therefore, the results of our study and setting up of BKV detection can be helpful for physicians to recognize at risk patients.
3. Patients and Methods
3.1. Patients
Urine samples of 122 kidney transplant patients were gathered from Ahvaz Golestan Hospital, southwest of Iran, during 2010-2011. The sample size was calculated using the statistical analysis based on the previous reports. All of our cases were immunocompromised renal transplant patients and there were no specific inclusion and exclusion criteria.
3.2. Sample Collection, DNA Extraction and Polymerase Chain Reaction
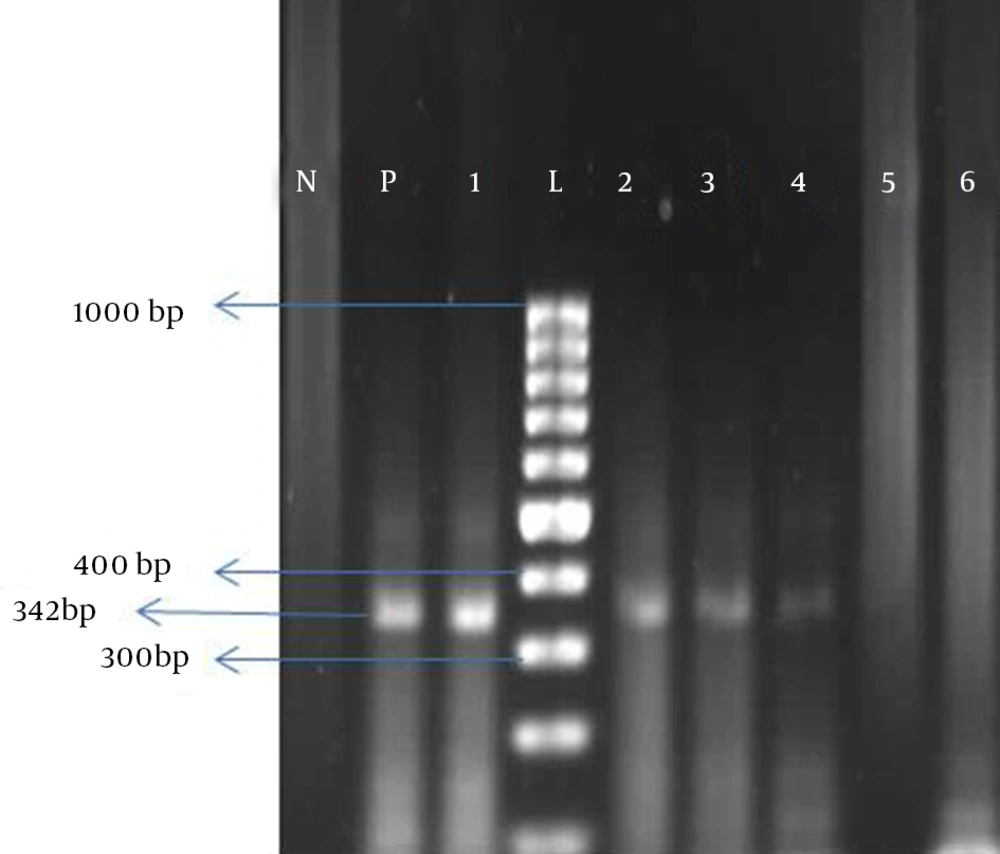
A 50-mL urine specimen was added to 0.5 mL of 0.5 Mol EDTA with pH = 8; all samples were sending to the infectious and tropical diseases research center of Ahvaz Jundishapur University of Medical Sciences. Each sample was centrifuged in 1500 rpm and 4°C for 10 minutes; the pellet kept in -20°C till test running time. Viral DNA was extracted using Roche high pure viral nucleic acid extraction Kit (REF. No11858874001 GmbH-Germany). Primers used to amplify the typing region were 327-1 PST 5'- gCC TgC AgC AAg TgC CAA AAC TAC TAA T-3' and 327-2 HIN 5'- gCA AgC TTg CAT gAA ggT TAA gCA TgC -3' Underlined nucleotides were added to create a PstI or HindIII cleavage site (8).
Extracted DNA was amplified using 5 μL DNA template, 0.3 μL Taq Polymerase, 1 μL dNTP, 0.25 μL each forward and reveres primers, 2 μL MgCl2 and 5 μL 10X PCR buffer, all mixed in a microtube and the ultimate volume reach to 50 μL using nuclease-free distilled water. For each run, we used Diethylpyrocarbonate (DEPC) water as a negative control and confirmed positive sample DEPC water as a negative control and confirmed positive sample from our previous study (9) as a positive control. After activation of reaction in 95°C for 5 minutes, amplification was performed for 50 cycles. Cycle program was as: 94°C for 60, 55°C for 60 and 72°C for 90 seconds. A final extension 72°C for 5 minutes was the ultimate step of cycles. Polymerase chain reactions (PCRs) were performed using the Peqlab thermocycler (Peq star, 96 Universal Gradient -Germany). The 342 bp amplification product was detected using 1.7% gel agarose socked in ethidium bromide (Figure 1). Positive samples for BKV were sent to Noor Genetic Laboratory in Ahvaz for sequencing. Sequences were analyzed using the neighbor Toning phylogenetic tree software and compared by reference ones.
3.3. Restriction Fragment Length Polymorphism
A 342bp which contained a 287bp BK type specific fragment, and which was amplified with PCR, treated by the restriction enzymes CfrI13 I, Alu I and Rsa I according to their package insert instructions. Table 1 shows fragment lengths resulted from restriction enzymes action on BK subtypes. One to 3 μL PCR product was added to 1-10 units of each enzymes, incubated in 37°C for 1 hour (If necessary overnight). Then digested products were separated using 3% agarose gel electrophoresis. The gels were visualized by ethidium bromide.
| I | II | III | IV | |
|---|---|---|---|---|
| Alu I | 193 | 193 | 342 | 342 |
| 149 | 149 | - | - | |
| Cfr13 I | 245 | 245 | 245 | 342 |
| 97 | 97 | 97 | - | |
| Rsa I | 294 | 342 | 212 | 342 |
| 48 | - | 130 | - |
4. Results
In this study, a total of 122 patients with kidney transplantation were admitted to Golestan Hospital in Ahvaz, southwest of Iran, for the determination of BKV infection using PCR. Considering that our study aimed to determine the epidemiological distribution of BKV, we couldn’t collect any information from our population using the questionnaire. Patients were from both sexes including 76 (62.3%) males and 46 (37.7%) females and also aged between 13-86 years (mean 38.6). From all study population, 51 (41.8%) of urine samples were positive for BKV DNA. Frothy-eight cases (94.11%) were subtype I and III others (5.89%) were subtype IV using the RFLP method. None of the patient’s urine samples were positive for subtypes II and III. Sequencing of positive samples confirmed the obtained results, which were shown that BK subtypes were positive for subtypes I and IV and any case was positive for subtypes II and III.
5. Discussion
BK virus belongs to the Polyomaviridae, which is able to infect the human. This virus may host in healthy persons. A frequency of 42% is reported for BKV in Chinese immunocompetent individuals (10). According to the current reports, infected donors could transport the virus to the recipients (11, 12). The BKV isolates worldwide are classified into four subtypes (I-IV) using serological and genotyping methods (4). Subtype I is widespread throughout the world, subtype IV shows a geographical distribution biased for East Asia, and subtypes II and III are rarely detected throughout the world (6, 7). In the current study, we have reported 41.8% frequency of the virus in our study population which is much higher than that of reported by Bohl et al. (13) that were about 8% and 13%. In a previous study, which was conducted by the author and colleagues in 1386, the prevalence of the virus in the urine of patients, were reported 38% (9). Chen and coworkers have reported 20%-30% frequency rates of BK-positive urines of south Asia immunecompromised individuals (14).
Our results show that the most frequent subtypes are subtype I and IV of BKV, respectively which is in accordance with other reports from other parts of world and from Iran. Such result proposes that till now the subtype I is the most frequent subtype worldwide. Nishimoto et al. (6) have declared that the origin of subtype IV is from Asia and is founded in variable rates in Asian populations. In turn Krumbholz et al. have reported that the most frequent BKV subtype in Germanic patients is a subtype I (15). Takasaka et al. have evaluated the prevalence of BKV in Japanese recipients of kidney or bone marrow transplantations; their results showed that the frequency rates of subtypes I, III and IV were 10%-20%, 70%-80% and 2%-3%, respectively (8). Ikegaya et al. have reported that the most frequent BKV subtype is a subtype I in different populations of Europeans (16). However, the prevalence of subtype IV was variable in different studies.
Chen et al. (14) study in south-east of Asia showed that in Japan and south-east parts of China, subtype I was more frequent than subtype IV; in north-east of China and Vietnam, subtypes I and IV had nearly equal frequencies but in Mongolian population subtype IV was the most frequent one (16). However, some epidemiologic investigations deny any significant dependency of BKV frequency to geographic distribution (15). The dependency of BKV frequency to geographical distribution is considerable about subtype IV and not considerable about subtype I, which is the most frequent subtype worldwide (8). Some studies have divided subtypes into subgroups and their population dependent frequencies are evaluated (8, 14, 15, 17, 18). Furthermore, Motazakker and collages have isolated only subtype I using RFLP from Uromia kidney transplant patients (19). According to the above-mentioned reports, although the patients with kidney transplantation who had BKV were more at risk than BKV negative patients, the different BKV subtypes didn’t affect the clinical outcomes in those patients.
The pathogenesis of BKV was discussed in many papers. When virus has reactivated, it spread by cell-to-cell (20-22). In some conditions that the immune system is out of control and the patients is immunocompromised a progressive lytic infection develops (23). Lysis of infected tubule cells results in viral leakage into the tubule lumen and urine. The spread of viral infection leads to necrosis of renal cells and its clinical and laboratory manifestations. Our work is the second study in Iran and considering huge numbers of transplantation in Iran and Khuzestan Province, southwestern of Iran, in addition to the role of this virus in kidney transplant rejection, a routine evaluation of BK positivity is recommended both for graft recipients and donors. This helps better transplantation results and may prevent graft rejection.