1. Background
Spinosyns, novel macrolides, are natural metabolites produced under aerial fermentation conditions by the actinomycete Saccharopolyspora spinosa. These compounds contain a unique tetracyclic ring system to which two different sugars are attached. Spinosad, a mixture of spinosyns A and D (Figure 1), is used as unique pesticide with a high selective activity against targeted pests and low toxicity in non-target organisms (including many beneficial arthropods). These characteristics make spinosad an excellent new tool for integrated pest management. The discovery and characterization of S. spinosa represents a novel opportunity to develop a portfolio of progressive insect-management tools from natural products (1-4).
In recent years, molecular and metabolic engineering methods have been widely employed to improve S. spinosa strains (5-10). However, classical strain-improvement methods, such as mutagenesis, are still the most efficient strategies for breeding many antibiotic-producing strains. We have focused on improving spinosad production of S. spinosa for several years. Even combined with high-throughput methods to screen nearly 20,000 strains, the dilemma we encountered was that no strains with spinosad production of > 500 μg/mL could be obtained with mutagenesis methods. We proposed that this limitation is probably caused by the composition of the production media.
Strobel et al. established a fermentation medium for screening high-titer mutants of S. spinosa (11). Other derived media were also reported for spinosad-production improvement (12-14). We evaluated these reported media with the strains in our lab, and observed no further improvements in spinosad production. The common factor of these media was that they all used glucose as their main carbon source.
2. Objectives
In the present study, a new fermentation medium was developed to enhance spinosad production in S. spinosa by systematically comparing the carbon-source utilizations. The media showed potential for use in future work after verification by several different strains.
3. Materials and Methods
3.1. Strains
Saccharopolyspora spinosa strain Co121, derived from wild-type strain NRRL 18395 through successive mutation induced by N-methyl-N'-nitro-N-nitrosoguanidine and 60Co radiation, was used in this experiment. The other strains used in this study were all derived from strain Co121.
3.2. Media and Growth Conditions
The S. spinosa strains were incubated on ATCC172 agar medium. Each liter of deionized H2O contained 10.0 g of glucose, 20.0 g of soluble starch, 5.0 g of yeast extract, 5.0 g of N-Z amine type A, 1.0 g of CaCO3, and 20.0 g of agar. The pH value was adjusted to 7.0 with 1 N NaOH before autoclaving at 30°C for 10 days to prepare the spore suspension. The spore suspension in 30% glycerol was stored at -80°C.
For the vegetative medium, each liter of deionized H2O contained 17.0 g of casein peptone, 3.0 g of soy peptone, 2.5 g of glucose, 5.0 g of NaCl, and 5.0 g of Na2HPO4. The pH value was adjusted to 7.0 with 1 N of HCl before autoclaving. Next, 100 μL of spore suspension was inoculated into 40 ml of vegetative medium in a 250-ml Erlenmeyer flask, then cultured for 48 hours on an orbital shaker at 30°C, 200 r/minute. Next, 2 mL of vegetative culture (5% v/v) was inoculated into 40 mL of fermentation medium in 250-mL Erlenmeyer flasks, and incubated on an orbital shaker for up to 8 days at 30°C, 200 r/minute. All media were sterilized by autoc1aving at 121°C for 20 minutes. The initial fermentation medium, per liter of deionized H2O, contained 40.0 g of glucose, 10.0 g of soy flour, 30.0 g of soluble starch, 10.0 g of corn steep liquor, 5.0 g of yeast extract, 10 g of cottonseed flour, and 3.0 of g of CaCO3. The pH value was adjusted to 7.0 with 1 N of NaOH before autoclaving.
3.3. Experimental Procedures
3.3.1. Carbohydrate Utilization Test
Media with various carbohydrates incorporated into a minimal medium with a final concentration of 10.0 g/L, for which each liter of deionized H2O contained 2.0 g of (NH4)2SO4, 0.2 g of MgSO4 7H2O, 0.5 g of NaH2PO4 H2O, 0.1 g of CaCl2 H2O, 0.5 g of K2HPO4, and 20.0 g of agar, were used to investigate the carbon sources utilized by S. spinosa. Next, 10 μL of the S. spinosa spore suspension was spread onto plates of different media, and growth was observed by incubating at 30°C for 10 - 14 days.
3.3.2. Carbohydrate Effects on Spinosad Biosynthesis
Strains were incubated in the minimal fermentation media, in which each liter of deionized H2O contained 10.0 g of soy flour, 10.0 g of corn steep liquor, 5.0 g of yeast extract, 10 g of cottonseed flour, and 3.0 g of CaCO3; pH was adjusted to 6.8 with 1 N of NaOH before autoclaving. The minimal medium was incorporated with utilizable carbohydrates to test the production of spinosad. The final concentration of carbohydrates was 60 g/L.
3.3.3. Optimization of Spinosad Production Media With Response Surface Design
Our previous results showed that cottonseed flour and corn steep liquor could significantly improve spinosad production (15). Therefore, the optimum carbon source, cottonseed flour, and corn steep liquor were chosen as the main factors for the response surface design to investigate effect on spinosad production. The other ingredients were kept constant. Response surface design and data analysis were carried out with Minitab® software (Minitab ver.15.1.30.0, Minitab Inc., USA).
3.4. Analysis of Spinosad Production
Spinosad production in liquid culture was analyzed by the HPLC method as previously described (16). Packed cell volume (PCV, % v/v) was determined by centrifugation of 10 mL of whole broth at 6000 × g for 30 minutes.
4. Results
4.1. Optimum Carbohydrates for Spinosad Production
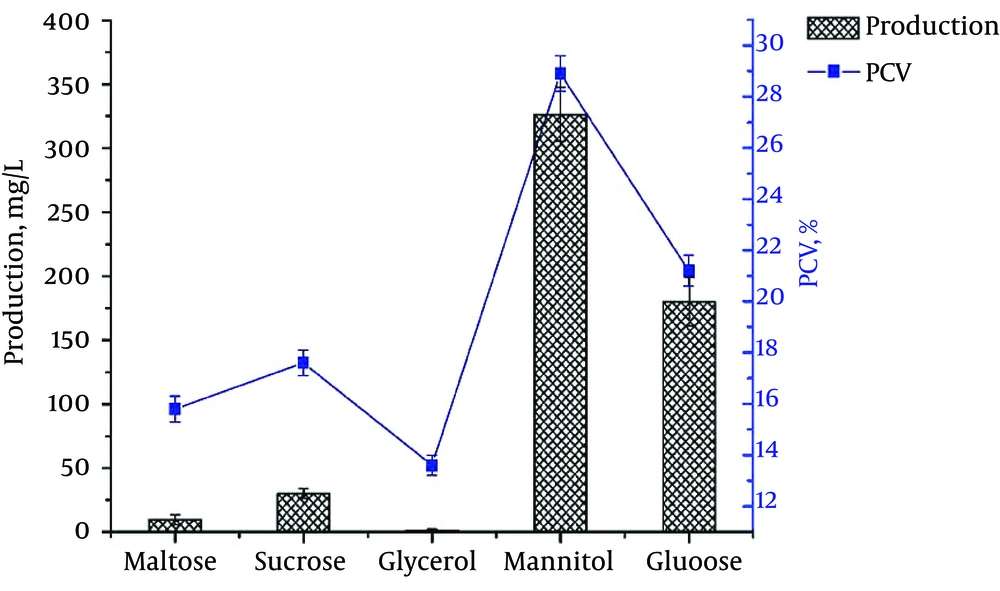
Glucose, lactose, mannitol, sucrose, sorbitol, glycerol, maltose, and soluble starch were evaluated for their utilization by S. spinosa in the minimal media. The results showed that S. spinosa could grow significantly more abundantly on media containing glucose, mannitol, maltose, sucrose, or glycerol than on media containing other carbohydrates as a solo carbon source.
Further experiments showed that mannitol and glucose significantly enhanced spinosad production in the fermentation media. Much less spinosad was produced in the fermentation media containing maltose and sucrose. There was nearly no spinosad production by S. spinosa Co121 in the fermentation medium containing glycerol as the main carbon source (Figure 2).
The highest biomass was achieved by the medium containing mannitol (28.9%). To verify whether mannitol was the optimal carbon source, the spinosad production of nine strains, all derived from Co121, was compared in fermentation media containing glucose or mannitol as the main carbon source, respectively. All strains could produce more spinosad in the medium containing mannitol. The average spinosad titer from the mannitol medium was 73.22% higher than from the glucose medium.
4.2. Optimizing Fermentation Medium With Response Surface Methods
The design and titers of spinosad are shown in Table 1 (average data from six replicate flasks). The results were analyzed with Minitab® and the multiple regression analysis for the total titers resulted in the following predication equation:

Where y = predicated total titer and X1, X2, and X3 = code levels of mannitol, cottonseed flour, and corn steep liquor. The regression coefficient for mannitol was significant (Table 2).
The response surface plot predicted that the titer reached a maximum of 549.96 μg/mL when the mannitol, cottonseed flour and, corn steep liquor were -0.15, 0.83, and 0.25, respectively. This suggested that the optimum concentrations of mannitol, cottonseed flour, and corn steep liquid were 98.0 g/L, 43.0 g/L, and 12.9 g/L, respectively.
| Run Order | Control Factor(Actual Concentration) | Titer, μg/mL | |||
|---|---|---|---|---|---|
| Mannitol | Cottonseed Flour | Corn Steep Liquor | Experimental | Predicted | |
| 1 | 0 (10.0 g/L) | 1 (44.0 g/L) | -1 (10.0 g/L) | 520 | 526 |
| 2 | 0 | 0 (38.0 g/L) | 0 (12.5 g/L) | 540 | 537 |
| 3 | 1 (115.0 g/L) | -1 (32.0 g/L) | 0 | 437 | 434 |
| 4 | 0 | -1 | 1 (15.0 g/L) | 496 | 490 |
| 5 | 0 | 0 | 0 | 529 | 537 |
| 6 | 1 | 0 | −1 | 462 | 457 |
| 7 | -1 (85.0 g/L) | 0 | 1 | 497 | 502 |
| 8 | -1 | 1 | 0 | 512 | 516 |
| 9 | -1 | 0 | -1 | 495 | 485 |
| 10 | 0 | 1 | 1 | 549 | 540 |
| 11 | 0 | −1 | -1 | 467 | 476 |
| 12 | 1 | 0 | 1 | 458 | 468 |
| 13 | 1 | 1 | 0 | 492 | 491 |
| 14 | -1 | −1 | 0 | 472 | 473 |
| 15 | 0 | 0 | 0 | 543 | 537 |
| Term | Coefficient | Standard Error | t-Value T | P |
|---|---|---|---|---|
| Constant | 537.333 | 6.377 | 84.266 | 0.000 |
| MAN | -15.875 | 3.905 | -4.065 | 0.010 |
| CF | 25.125 | 3.905 | 6.434 | 0.001 |
| CSL | 7.000 | 3.905 | 1.793 | 0.133 |
| MAN^2 | -44.542 | 5.748 | -7.749 | 0.001 |
| CF^2 | -14.542 | 5.748 | -2.530 | 0.053 |
| CSL^2 | -14.792 | 5.748 | -2.573 | 0.050 |
| MAN+CF | 3.750 | 5.522 | 0.679 | 0.527 |
| MAN+CSL | -1.500 | 5.522 | -0.272 | 0.797 |
| CF+CSL | -0.000 | 5.522 | -0.000 | 1.000 |
Abbreviations: CF, cottonseed flour; CSL, corn steep liquor; MAN, mannitol.
4.3. Verification of Optimized Medium
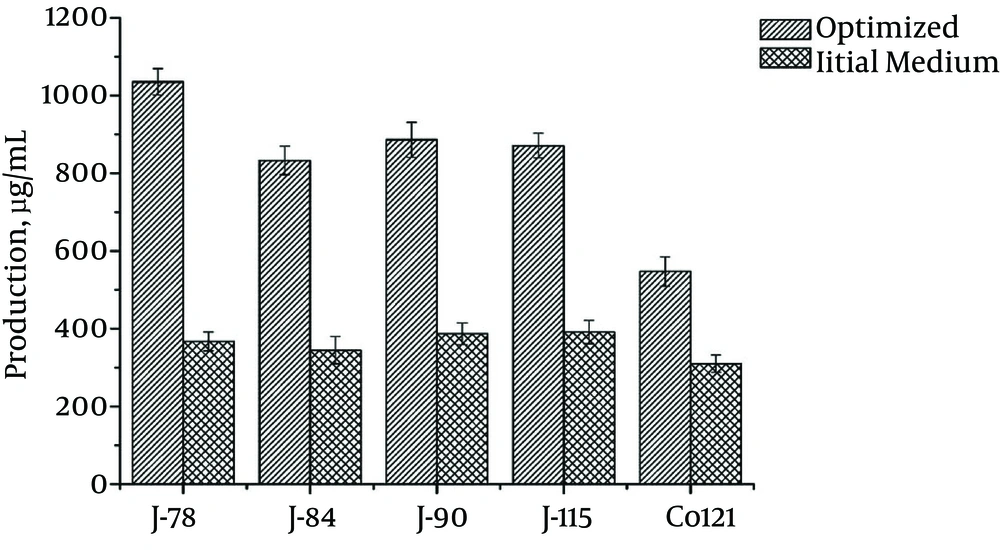
The optimum fermentation medium composition determined by the response surface model was mannitol (98.0 g/L), cottonseed flour (43.0 g/L), corn steep liquid (12.9 g/L), KH2PO4 (0.5 g/L), and CaCO3 (3.0 g/L), with a pH of 7.0 before autoclaving. The spinosyn production of nine replicated shake-flask cultures of S. spinosa Co121 grown in the optimized fermentation medium was 547 μg/mL with a standard deviation 38 μg/mL (Figure 3). The results verified the 549.96 μg/mL level predicted by the response surface plot.
After testing 4,000 mutants from strain Co121 through a high-throughput screening system for S. spinosa (17) with the optimized medium, we obtained four strains with higher spinosad production (Figure 3). Compared with production in the initial medium, the spinosad titer of the highest-producing strain, J-78, increased 2.82-fold. The overall increase in the spinosad titer, from strain Co121 in the original fermentation medium to the mutant strain J-78 (1035 ± 34 μg/mL) in the optimized medium, was 3.33-fold.
5. Discussion
Wang et al. used intergeneric protoplast fusion between S. spinosa and Streptomyces avermitilis to improve the starch-utilization problem (18). Huang et al. proved that maltose-transport-related genes obviously promoted the growth of S. spinosa and resulted in improved spinosad yields (19). All of these studies showed that the bad utilization of starch or maltose by S. spinosa results from a bad maltose-transfer system. The medium we developed, which contained mannitol as the main carbon source, probably avoided this system and improved the production of spinosad. This medium showed potential as a candidate for further strain improvements. This study also showed that the response surface method is an efficient technique for optimization of media for antibiotic-producing strains.