1. Background
Aflatoxins are the most extensively studied group of mycotoxins produced by molds, especially the Aspergillus group (Aspergillus. parasiticus, A. flavus, A. nomius), which are highly toxic to animals and humans (1). Aflatoxin M1 (AFM1) is the main hepatic carcinogenic metabolite of aflatoxin B1 (AFB1) with weaker carcinogenicity compared to AFB1 (2, 3). AFM1 appears initially in animal tissues and biological fluids (milk and urine) after intake of foods contaminated with AFB1 and its subsequent conversion into AFM1 through hydroxylation (4, 5).
Considering that milk and dairy products constitute an essential component of human diets and AFM1 is heat-stable and remains by milk pasteurization, sterilization, or freezing, ingestion of dairy products is the primary route of AFM1 entering into the body. Therefore, early detection of AFM1 in dairy products may act as an appropriate alarm indicating the possibility of health hazard (6, 7). Many countries have performed studies about the incidence of AFM1 in milk and dairy products (8-10). Despite the fact that specific antibodies against AFM1 have been prepared and various immunoassays have been developed for detection of the toxins in food (11-20), there is still a need for a fast, reliable, and more sensitive analytical method for quantifying small amounts of AFM1.
There are a variety of chromatographic methods commonly used for the analysis of AFM1 and other aflatoxins in many different foods such as thin-layer chromatography (21), high-performance liquid chromatography (22, 23), immunoaffinity chromatography combined with high-performance liquid chromatography (24), and overpressured layer chromatography (25). Although these methods are sensitive, they are time-consuming and require complicated instrumentations (26, 27). Since immunoassay provides a simple and rapid method for the analysis of many toxic substances in comparison to the methods mentioned above (28, 29), it is necessary to design specific and sensitive antibodies for AFM1 detection. In the present study, we describe efficient conjugation of AFM1 to Bovine Serum Albumin (BSA) through AFM1-(O-carboxymethyl) oxime derivative and we also attempt to produce rabbit anti-AFM1 antibodies with proper specificity and purity. This antibody can then be used to develop an ultrasensitive ELISA kit for detection of AFM1 in milk and other biological fluids.
2. Objectives
The current study was conducted to produce bioconjugate of AFM1 with BSA as well as to generate specific antibodies against AFM1 for immunoassay of the mycotoxin.
3. Materials and Methods
3.1. Preparation of AFM1-(O-carboxymethyl) Oxime Derivative
Hsu and Chu (14) reported a useful method for coupling aflatoxin to protein. Here, we intend to describe a modification of the method proposed by Hsu and Chu. Since AFM1 lacks a reactive group to conjugate with carrier proteins such as BSA, AFM1 (Sigma, USA) was converted to AFM1-(O-carboxymethyl) oxime (AFM1-oxime) as follows: 152 μg of O-(Carboxymethyl) hydroxylamine hemihydrochloride (Sigma, USA) in 1 mL of deionized water was mixed with 100 μg of AFM1 in 4 mL methanol, and then 1 mL of pyridine was added to the mixture. The mixture was refluxed for 3 hours and incubated at room temperature for an overnight. After reaction, the solvents were removed by rotary evaporator. The reaction product (AFM1-oxime) was orange yellow in color, possessing a reactive carbonyl group and able to react with the amine groups of proteins.
The reaction product and aflatoxin standard were dissolved in chloroform and analyzed by thin-layer chromatography (TLC). TLC was performed by spotting the product and aflatoxin standard near the base of a silica gel plate followed by developing the TLC plate in acetone-chloroform (1:9). In addition, the product of the reaction was analyzed by Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) using a ODS column (150 mm × 4.6 mm). AFM1 at the concentration of 10 ppb in methanol (as standard), carboxymethyl hydroxylamine at the concentration of 10 ppb in water and AFM1-oxime at the concentration of 10 ppb in methanol were prepared. About 20 μL of each sample and standard were injected into the HPLC system. Isocratic solution of methanol-acetonitrile was used as the mobile phase. Elution was monitored by a spectroflurometric detector (RF-551, Shimadzu, Kyoto, Japan) operated at excitation and emission wavelengths of 365 and 420 nm, respectively. The column oven temperature was kept constant at 35°C and the mobile phase was filtered, degassed and pumped at a flow rate of 2.2 mL/min.
3.2. Conjugation of AFM1-(O-carboxymethyl) Oxime to Bovine Serum Albumin
One hundred microliter of AFM1-oxime in 400 μL of 25% ethanol containing 6.5 mg EDS was mixed with 200 μg of BSA (Sigma, St. Louis, MO, USA) in 20 μL of 0.05 M sodium phosphate buffer (pH 7.2). The reaction mixture was stirred at room temperature for 48 hours in complete darkness. Two additional 6.5 mg portions of EDS were added to the reaction mixture during this period. After reaction, the mixture was dialyzed against deionized water for 24 hours, with 3 times water change, followed by centrifuging at 10000 × g for 10 minutes. The conjugation of AFM1-oxime to BSA was analyzed by UV-VIS spectrophotometry against pure BSA.
3.3. Immunizations
Female rabbits, 6-8 weeks old, were obtained from Razi Institute (Tehran, Iran) and immunized with the AFM1-BSA conjugate as follows: about 150 μL of AFM1-BSA conjugate (500 μg/mL) was emulsified with equal volume of Complete Freund's adjuvant (CFA, Sigma) for the first injection and Incomplete Freund's adjuvant (IFA, Sigma) for the booster injections with 2-weeks intervals. The first injection was performed intradermally at 5 points on the animal’s back and sides. The booster injections were introduced intramuscularly. A normal rabbit was immunized with BSA as described previously. Starting at 3 or 4 weeks after the initial immunization, rabbits were bled on biweekly intervals and antibody titers were determined by an indirect ELISA. Rabbits were bled 3 weeks after final injection, the time when desirable titers of antibody were found. Antiserum was separated from the clot by centrifugation at 4000 × g (4°C) for 30 minutes and purified as follows.
3.4. Purification of IgG Fraction of Rabbit Serum
The serum was diluted thrice with PBS and half saturated (50%) with ammonium sulfate. The precipitated fraction was separated by centrifugation and dialyzed against 100 mM Tris-HCl buffer pH 7.8 and purified by a DEAE-Sephacel (Amersham Pharmacia Biotech) column equilibrated with the same buffer.
3.5. Affinity Chromatography With Bovine Serum Albumin Ligand
For further purification of BSA-AFM1 antibody, the antibody produced against AFM1 was negatively selected by a BSA-Sepharose 4B affinity column. In order to prepare affinity medium, CNBr-activated Sepharose 4B (Amersham Pharmacia Biotech) was swelled in adequate 1 mM HCl for two hours. For each milliliter of the swelled resin, 5 mg of BSA in 0.1 M carbonate buffer, pH 8.5 containing 0.5 M NaCl was added and the mixture was stirred for 2 hours at RT. Residual active sites were blocked with 0.2 M glycine containing 0.5 M NaCl. The resin was washed thrice with alternate buffers (50 mM acetate buffer pH 4 and 50 mM carbonate buffer pH 9 containing 0.5 M NaCl), then loaded on to a column and equilibrated with phosphate buffered saline (PBS) pH 7.2. The purified IgG fraction at concentration of 5 mg/mL was applied to the column, and the column was washed with PBS till the absorbance of effluents reached zero at 280 nm. Finally, 3 column volumes of 0.1 M glycine with pH 2.7 were used to elute bound antibodies from the column.
3.6. Enzyme-linked Immunosorbent Assay
ELISA method was used to determine the titers of prepared antiserums and purified antibodies as well as the cross reaction of purified antibodies with other aflatoxins (AFB1, AFB2, AFG1, AFG2). The optimal dilution of antigen pre-coated on the microtiter plate was determined by a checkerboard test. One hundred microliter of antigen (AFM1-BSA or BSA at 5 µg/mL in PBS) was coated for 2 hours at 37°C. After 3 times of washing with PBS containing 0.05% (v/v) Tween 20 (PBS-T), nonspecific sites were blocked with PBS-BSA 2% (w/v) for 2 hours followed by 2 washes with PBS-T. 100 µL of 1:1000 to 1:64000 dilutions of the antiserums, purified antibodies or standard antiserums were added to each well and incubated for 1 hour at 37°C. The plates were washed 5 times with PBS-T and 100 µL of 1:10000 dilutions of secondary antibodies (goat anti-rabbit IgG conjugated with HRP, prepared in our laboratory) was added to each well. Plates were incubated for 45 minutes at 37°C and after 5 times of washing, 100 µL of TMB substrate solution (Sigma, St. Louis, MO, USA) was added. After 20 minutes of incubation in a dark place, the reaction was stopped with 100 µL of 5% solution of sulfuric acid. Absorbance of wells was read by a plate reader (Stat Fax 2100, Awareness Technology Inc. Palm City, USA) at 450 nm.
3.7. Analytical Methods
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) was performed using 12.5% separating and 4% stacking gels containing 0.1% SDS (30). Protein samples were heated in boiling water for 5 minutes and resolved at 50 V for 30 minutes and 150 V for 1 hour followed by Coomassie Blue R250 staining. The protein concentrations were determined by the method of Bradford (31) using BSA as the standard protein.
4. Results
4.1. Preparation of Aflatoxin M1-(O-carboxymethyl) Oxime
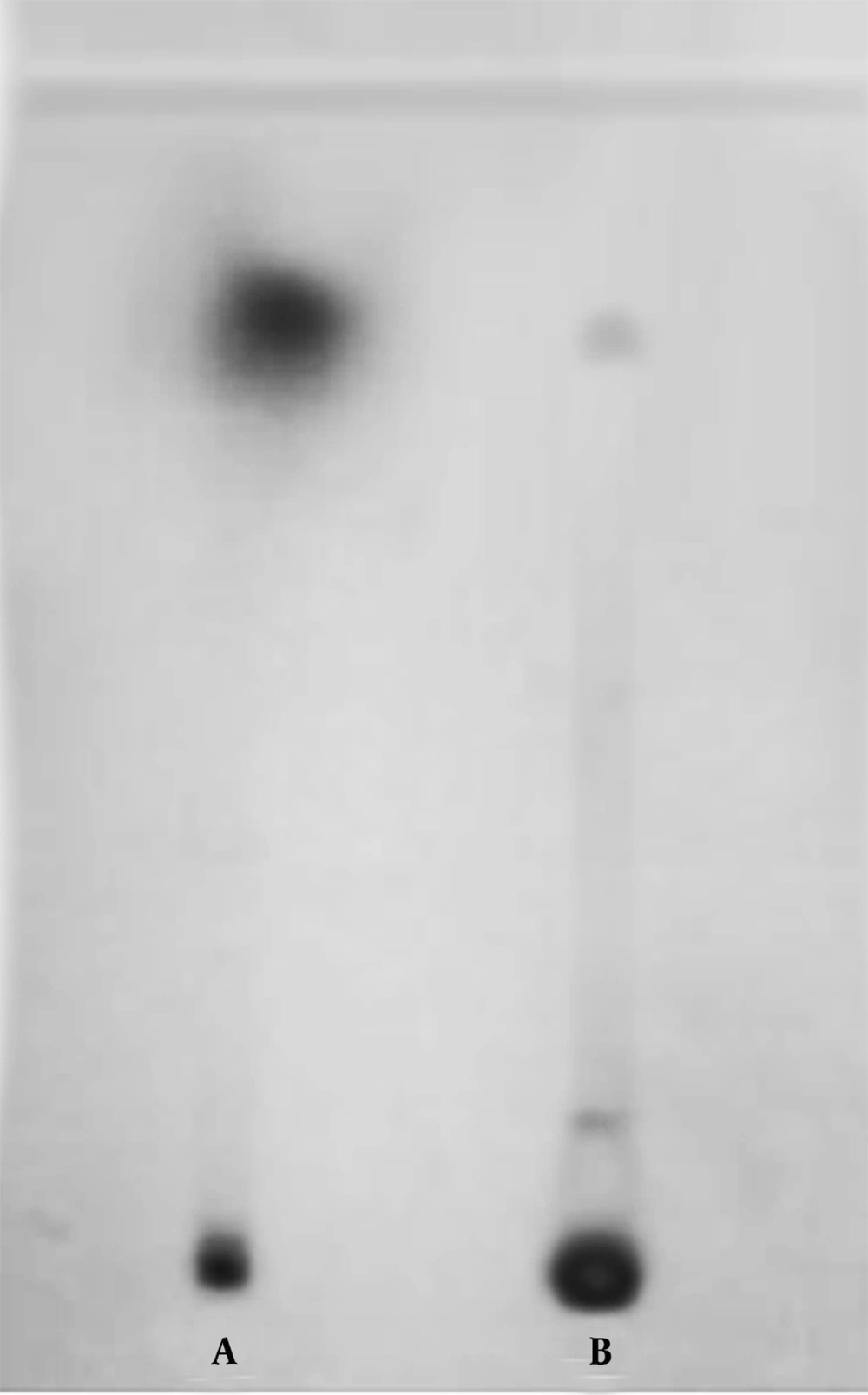
In order to confirm the coupling of AFM1 to O-(Carboxymethyl) hydroxylamine hemihydrochloride, the reaction product was analyzed by TLC and RP-HPLC. Analysis of the product by TLC plate developed in acetone-chloroform (1:9) yielded two spots, using UV detector (Figure 1). The minor spot in the upper part of the gel had the same Rf value as the standard aflatoxin (Figure 1 lane A), and the major spot with lower Rf value was the AFM1-oxime derivative (Figure 1 lane B).
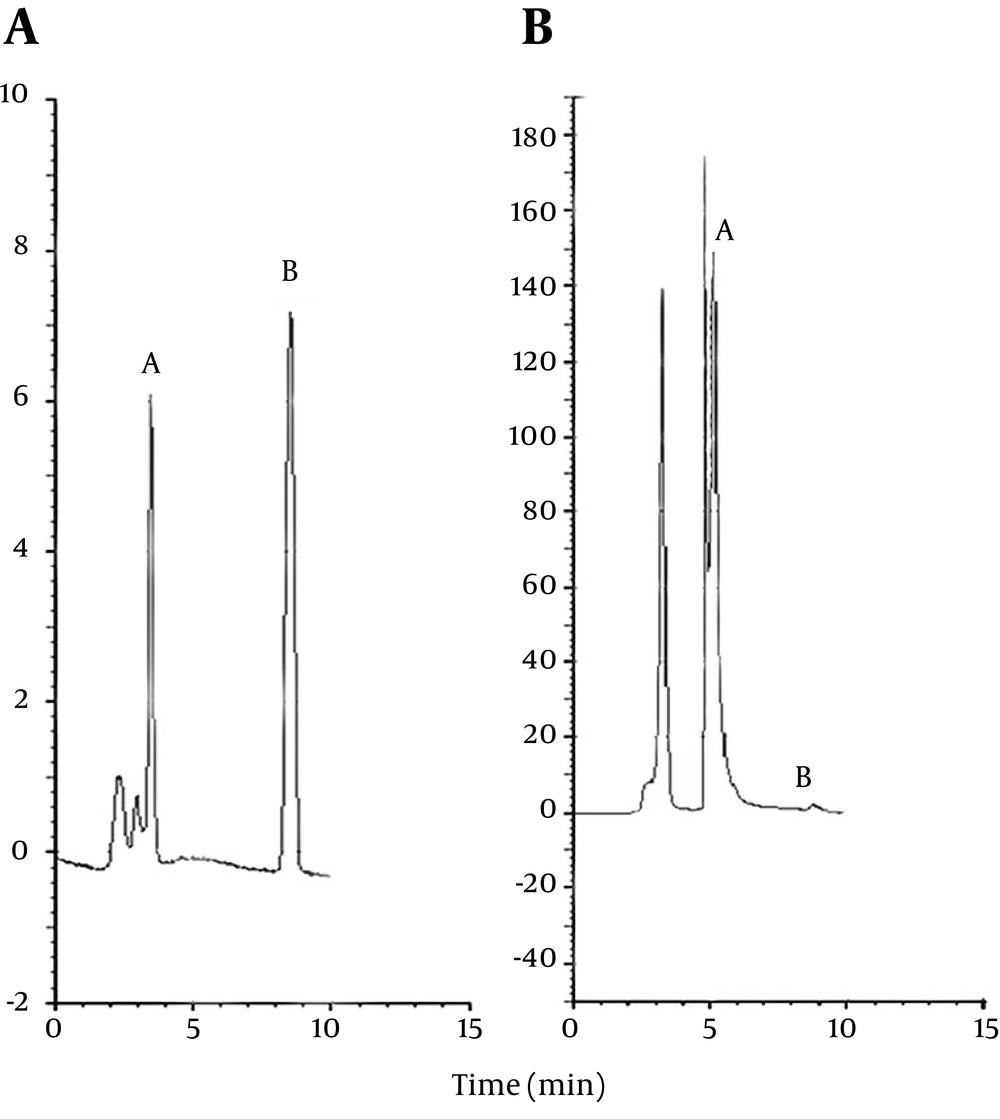
For further confirmation of the AFM1 coupling to O-(Carboxymethyl) hydroxylamine hemihydrochloride, the standard aflatoxin and the reaction product at the concentrations of 10 ppb were injected into the RP-HPLC system and retention times were measured. Figure 2 A shows the pattern of standard aflatoxin (retention time 9 minutes, peak B). The other peaks in this Figure correspond to the solvent. Figure 2 B shows the pattern of reaction product, which includes a major peak (retention time 6 minutes, peak A) and a minor peak (peak B) that eluted at the same retention time as standard aflatoxin peak. Peak B, which is negligible, corresponds to the free aflatoxin. By injection of O-(Carboxymethyl) hydroxylamine into the HPLC system, no peak appeared, indicating that it lacks any absorbance at the wave length of 365 nm and emission wavelength of 420 nm. Therefore, the peak eluted at 6-minute retention time corresponds to the AFM1-oxim conjugate (peak A in Figure 2 B).
4.2. Conjugation of Aflatoxin M1-(O-carboxymethyl) Oxime to Bovine Serum Albumin
The UV spectrum of the reaction product from the wave length of 200 - 800 nm showed two peaks (Figure 3). The first peak at the wavelength of 280 nm corresponds to BSA. The peak at the wavelength of 365 nm corresponds to the coupled reaction product. The free AFM1 molecule was separated from the reaction product by dialyzing against deionized water, so the peak at 365 nm corresponds to BSA-AFM1 conjugate free of AFM1 contaminants. The results obtained from spectrometric method indicated that about 12 moles of AFM1-oxime were successfully coupled to 1 mole of BSA.
4.3. Preparation and Purification of Anti-AFM1 Antibodies
After each injection of antigen (BSA- AFM1) to rabbit, the anti-AFM1 antibody levels of the sera were monitored using a designed indirect ELISA. According to the ELISA results, the antibody titers were relatively weak after first and second injections and desirable titers were obtained 2 weeks after the third injection. The highest titers were observed after the fourth injection. Rabbits were bled two weeks after the last injection, when the maximum titers of antibody (64000 times) were found and the antiserum was prepared. The titers of prepared anti-AFM1 antiserum were compared to the antiserum of immunized rabbit with standard BSA-AFM1 conjugate and it was found that the titers of prepared antiserum in this study were suitable for use in immunological assays for the analysis of AFM1.
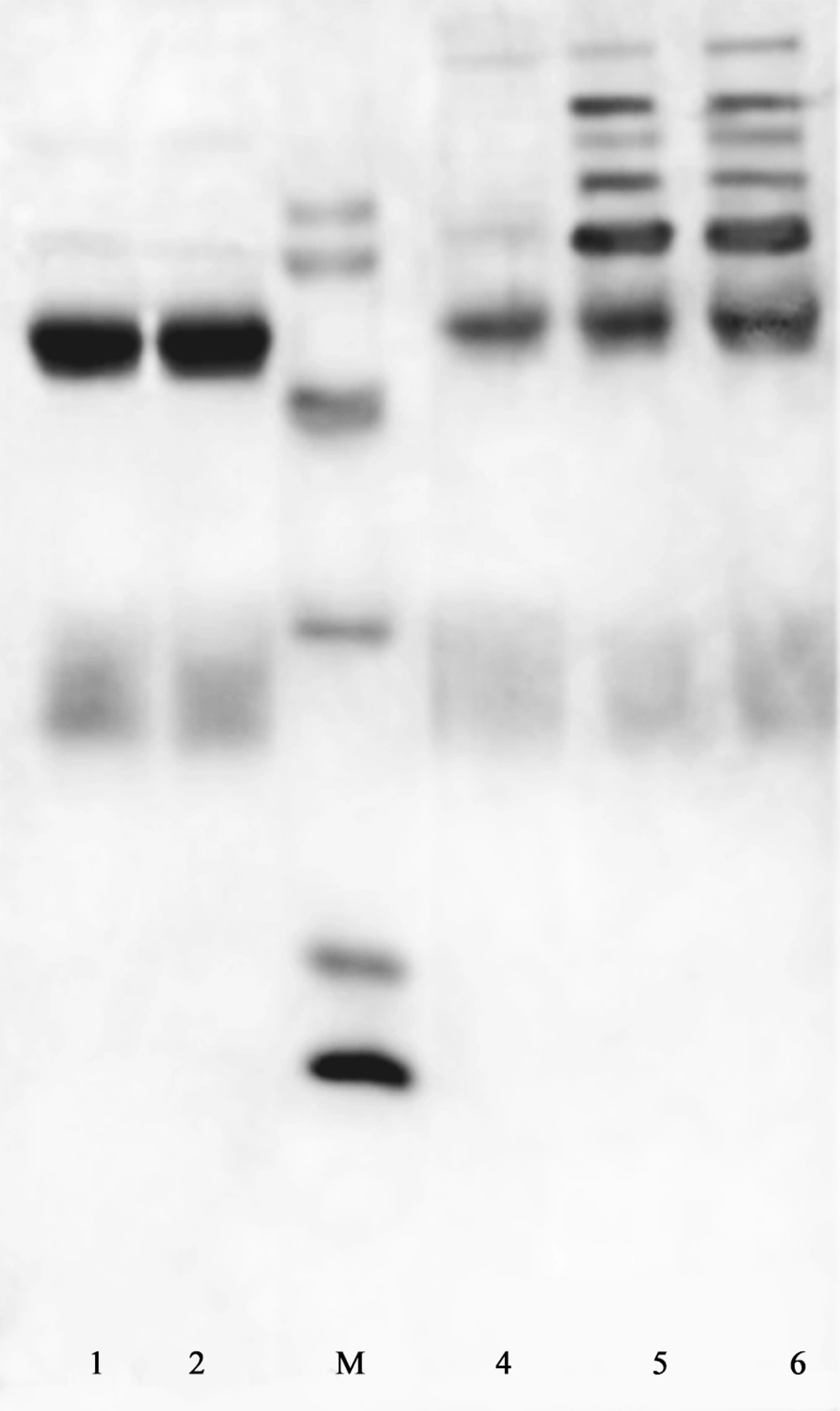
The purity of prepared antibodies was estimated by SDS-PAGE (Figure 4). Ammonium sulfate fractionation caused partial purification of the antibody (Figure 4, lane 6). The first peak obtained from ion exchange chromatography contained IgG fraction with high purity. Reducing SDS-PAGE of this fraction included two intense bands at the MW of 26-27 and about 50 KDa, corresponding to the light and heavy chains of IgG, respectively (Figure 4, lanes 1-2). In Figure 4, lanes 4 and 5 show the protein content of second and third peaks of ion exchange chromatography, respectively that have been eluted from the column, with equilibrium buffer containing 0.1 and 1 M NaCl. They contained other impurities in addition to some parts of IgG.
In order to increase the specificity of antibody against AFM1 and excluding anti-BSA antibodies, we used BSA-Sepharose 4B affinity chromatography. An indirect ELISA, using BSA or BSA-AFM1 (5 µg/mL) as the coated antigen, was conducted to ensure that the antibody produced against protein carrier was completely removed by the BSA-Sepharose 4B affinity chromatography. The results showed that the column unbound antibody is very reactive and specific against BSA-AFM1 (OD; 1 at 1:32000 dilution) and not reactive to BSA at the same dilution. These findings clearly show that the column unbound antibody includes specific antibody against AFM1 and was successfully separated from the anti-BSA antibody by the affinity chromatography using BSA ligand.
4.4. Determination of Antibody Specificity
Specificity of purified antibodies obtained from rabbits immunized with BSA-AFM1 was determined by a competitive ELISA. The antibody used in the assay was the BSA-Sepharose 4B affinity purified antibody. Results showed that the purified antibodies have the highest affinity to AFM1 in comparison to related aflatoxins. Concentrations required to decrease absorbance by 50% (or to displace the bound AFM1-proxidase) in the presence of different aflatoxins were 1, 70, 105, 240, 2500 ng/mL for AFM1, AFB1, AFB2, AFG1, and AFG2, respectively.
5. Discussion
In the present study, we described an optimized method for the preparation of BSA-AFM1 conjugate through formation of AFM1-(O-carboxymethyl) oxime derivative and polyclonal antibody against AFM1. Aflatoxin M1, like many other aflatoxins is an organic compound with low-molecular weight, which is not sufficiently immunogenic to elicit an immune response alone. The toxin can be made immunogenic by conjugation to a suitable carrier. Since AFM1 also lacks a reactive group to conjugate with a macromolecule carrier (through derivatization), a free carboxylic group was introduced to the toxin molecule and AFM1 was converted to AFM1-(O-carboxymethyl) oxime (AFM1-oxime) through the method described by Hsu and Chu (14) with some modifications. Notably, preparation of AFM1-protein conjugates is expensive (AFM1, € 8000.00 per mg) and harmful to the operators. Therefore, we developed some modifications and could produce AFM1-BSA conjugates only with 0.1 mg AFM1. In our method by increasing of solvent volumes, the number of effective contacts between reactants decreased and the reaction was carried out over a longer period.
The results obtained from TLC showed that the product (AFM1-oxime) has more polarity than aflatoxin due to its free carbonyl group and travels a shorter distance on the silica gel plate compared to nonpolar one. Thus, our findings indicate that the reaction progressed well towards the product. The amount of unreacted aflatoxin was negligible and separated from AFM1-oxime conjugate by dialysis. Subsequently, AFM1-oxime was conjugated to BSA. Results obtained from spectrometric method showed that 12 moles of AFM1-oxime were coupled to 1 mole of BSA. Since immunoassay provides a simple and rapid method for the analysis of AFM1 in comparison to high-performance liquid chromatography and thin-layer chromatography, it is necessary to produce antibody against AFM1 with higher purity and specificity than those of the previous reports. The procedure of producing specific antibodies against aflatoxin M1 has been described and many research groups have also developed several enzyme immunoassays for AFM1 detection in milk and milk products (11-18).
The titers of prepared antibody were comparable to common standard anti-AFM1 antibodies. The antibodies obtained from rabbits immunized with BSA-AFM1 were purified by ammonium sulfate fractionation and ion exchange chromatography. In order to remove anti-BSA antibodies and increase the specificity, we also used BSA-Sepharose 4B affinity chromatography. Since the purified antibody by ion exchange chromatography contains both antibodies against AFM1 and BSA, the affinity chromatography using BSA ligand is a proper method for removing the antibody produced against the protein carrier. Results obtained from ELISA showed that the antibody resulting from affinity chromatography has proper affinity against BSA-AFM1 while the interaction of that with BSA at the same dilution is negligible. The findings clearly showed that the unbounded antibodies to the column included a specific antibody for AFM1 and was successfully separated from the anti-BSA antibodies by affinity chromatography using BSA ligand.
The specificity of purified antibody competition to AFM1 was determined by direct ELISA with AFM1-proxidase as the marker. The results showed that the purified antibodies had the highest affinity to AFM1 and the relative cross-reactivities of toxins (relative to AFM1) with purified anti-AFM1 antibody, as determined by the amount of aflatoxin necessary to cause 50% inhibition of enzyme activity were 70, 105, 240, 2500 ng/mL for AFB1, AFB2, AFG1, and AFG2, respectively. Thus, the inhibition of binding prepared anti-AFM1 antibody to aflatoxin M1 by different aflatoxins is negligible. The higher specificity of the prepared antibody against AFM1 compared to previous reports is due to the proper conjugation and removal of the antibody produced against BSA by BSA-Sepharose 4B affinity chromatography.
In conclusion, we proposed a useful method for preparation of AFM1-oxime and conjugation of AFM1-BSA at a proper molar ratio. Moreover, we produced rabbit antibody against AFM1 with high purity and specificity; this antibody can be used for development of ultrasensitive ELISA kits to assay AFM1 in biological fluids.