1. Background
Human T-lymphotropic virus type Ι (HTLV-Ι) was first identified in humans in 1980 (1) and 1982 (2). A type C retrovirus, HTLV-Ι, is the causative agent of two distinct human diseases, adult T-cell leukemia (ATL) or lymphoma, and a chronic progressive demyelinating disorder known as HTLV-Ι-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (3). It is estimated that 10 to 20 million people worldwide are infected with HTLV-Ι (4). HTLV I and II are transmitted through breastfeeding, sexual contact and blood transfusion. Transplacental transmission is also suspected (5, 6). Whole blood components, platelets and packed red blood cells, but not fresh frozen plasma, are sources of virus transmission (7). The probability of seroconversion in a recipient of contaminated blood is about 44% (8).
Patients with thalassemia need infusion of four to six blood units per month and therefore the risk of blood-borne diseases caused by viruses such as HTLV-I, hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV) increase in these patients (9). The association between retroviruses and hematologic malignancies has also been described previously. However, few studies on the association between HTLV-Ι infection and malignancy risk have been published (10). There are some case reports regarding finding HTLV-Ι in lymphoid malignancies except ATL (11). It is still controversial whether or not HTLV-Ι infection affects the incidence of several malignancies. Adedayo et al. found an association between HTLV-Ι and lymphoid malignancies in the Dominican population (12). Most of HTLV-I-infected individuals remain asymptomatic throughout their lives and in a few subjects HTLV-I-associated diseases will appear.
This infection is endemic in southern Japan, the Caribbean Basin, central Africa, central and south America, the Melanesian Islands in the Pacific Basin, and in the aboriginal population in Australia (13). In Iran, this virus has been found in isolated pockets in which HTLV-Ι infection is endemic (Khorasan, the northeastern province of Iran). The prevalence of HTLV-Ι infection in Mashhad, Khorasan was 0.77% among seeming healthy blood donors (14). However, we have few data about the prevalence of HTLV-Ι and HTLV-II in patients with hematological disorders and malignancies in Iran. According to another reports, the prevalence of HTLV-I infection in Mashhad, Neishbour, and Sabzevar was 2.1%, 3% and 1.6%, respectively (15, 16). However, the virus is less frequent in other parts of Iran including Urmia (0.34%) in northwest and Chaharmahal and Bakhtiari (0.62%) in southwest of Iran (17, 18).
2. Objectives
The aim of this study was to determine the prevalence of HTLVs among patients with chronic myelogenous leukemia (CML), acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), lymphoma, hemophilia and thalassemia, and hemophilia. Therefore, this study was conducted to detect HTLV-Ι and HTLV-II in patients with hematological disorders from Isfahan province, center of Iran.
3. Patients and Methods
3.1. Population Study
In this cross-sectional study, 101 patients with confirmed hematological disorders admitted to the oncology and thalassemia unit of Seyed Al-Shohada hospital, Isfahan, Iran, were enrolled during April to October 2012. Diagnoses of the diseases were confirmed based on previous clinical and laboratory document findings.
3.2. Sampling
Hematological disorders were as follows; leukemia (20 cases), lymphoma (four cases), thalassemia (67 cases) and hemophilia (10 cases). About 5 mL blood was collected from each patient without adding any anticoagulant. Serum was stored at -20°C before testing.
3.3. DNA Extraction
DNA was extracted from each 100 μL blood serum using high pure extraction kit (CinnaPure cell DNA kit, Cat No. PR881613-50, Iran).
3.4. Polymerase Chain Reaction
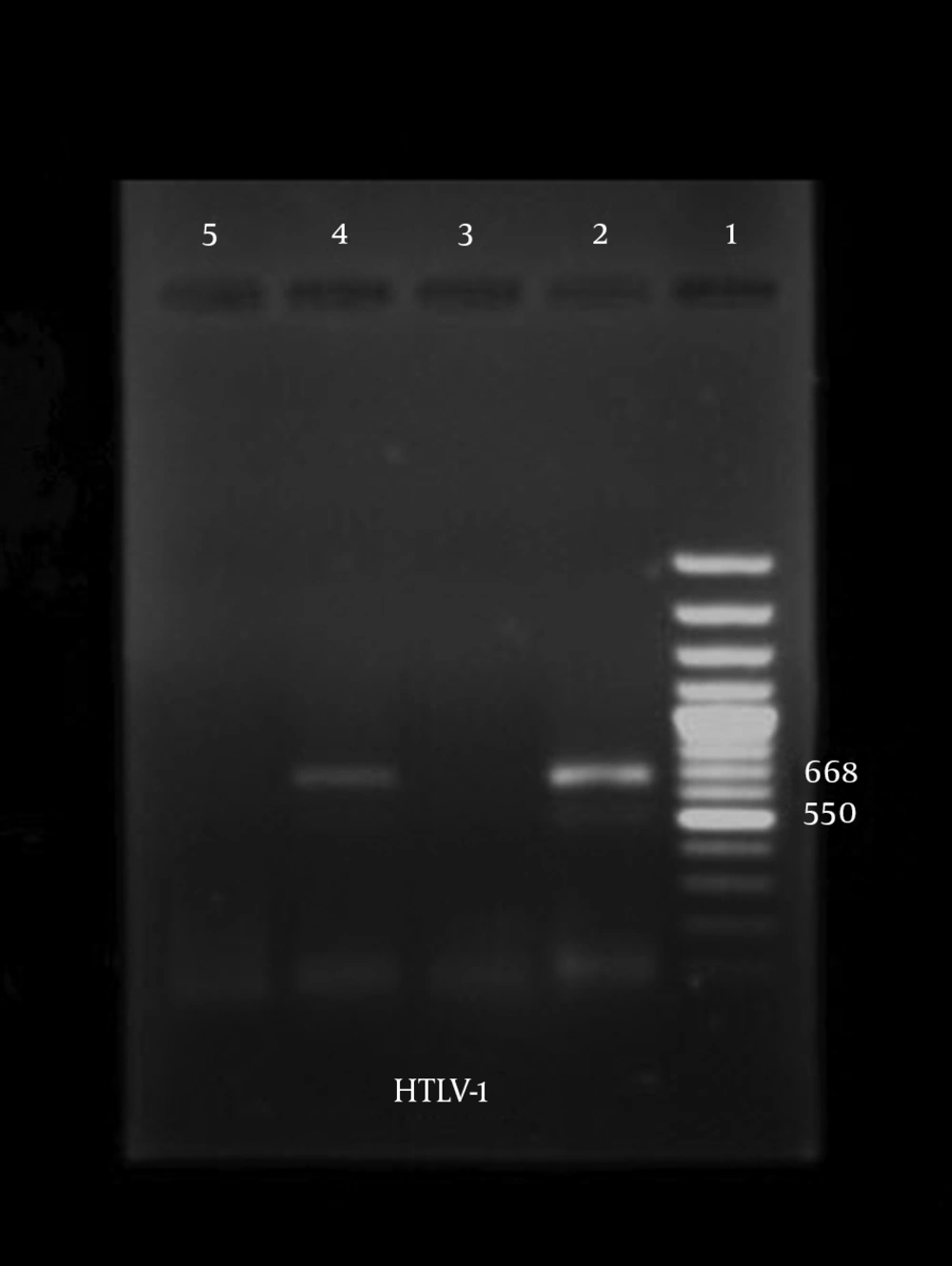
PCR amplification was performed with two sets of primers, pol (forward, CCCTACAATCCAACCAGCTCAG; reverse, TGGAGTAACTTACTAGGTTAG) for HTLV-Ι (GenBank accession No. J02029); and tax (forward, CGATTGTGTACAGGCCGATTG; reverse, CCTGTACACCAGGCAGTCTGGA) for HTLV-ΙΙ (GenBank accession No. M10060); resulting in amplicons of 668 bp and 628 bp, respectively, according to Dehee et al. (19) guidelines.
The total PCR reaction volume was 25 µL. It contained 5 µL DNA sample, 1 µL of each primer (10 pmol, TAG Copenhagen, Denmark), 0.5 µL Taq DNA polymerase (500 U،TA7506C CinnaGen, Iran), 2.5 µL PCR buffer 10x, 1 µL dNTP mixture (10 mM, DN7603C, CinnaGen, Iran), 1 µL MgCl2 (50 mM, TP7506C, CinnaGen, Iran) and 13 µL distilled water. For HTLV-Ι, the reaction mixture was incubated for five minutes at 95°C and then subjected to 40 cycles consisting of 30 seconds at 94°C, 30 seconds at 57°C, and one minute at 72°C, and a final extension for seven minutes at 72°C in a DNA thermal cycler (Corbett, Research, CGI-960, Australia). For HTLV-ΙΙ, the reaction mixture was incubated for five minutes at 95°C and then subjected to 40 cycles consisting of 30 seconds at 94°C, 30 seconds at 62°C, and one minute at 72°C, and a final extension for seven minutes at 72°C. Genomic DNA of HTLV-Ι-infected cell line, TL-Om1, was used as positive control. Distilled water was used as negative control. The PCR products were analyzed by electrophoresis on agarose gel (1%) and stained with ethidium bromide.
4. Results
One hundred and one patients with confirmed hematological disorders were recruited in this study. The mean age of patients was 44.25 years, ranging from 1.5 to 87. Of 101 patients, 67 (66.33%) and 34 (33.66%) were female and male, respectively. According to the type of hematological disorders, the most cases with thalassemia were in the age group of 20 - 40 years (Table 1). Most of the patients with leukemia (20) were in the age group of 1 - 6 years and in hemophilic cases (10), most of the patients were in the age group of 40 - 60 years. In the lymphoma group (4), all of the four cases were over 12 years. The results showed that only one male (0.99%) was positive for HTLV-I in patients with thalassemia. According to the results of PCR, the overall prevalence of HTLV-I infection in patients with thalassemia in Isfahan was 1.49%. All the samples were negative for HTLV-II (Figure 1).
| Age, y | Lymphoma | Hemophilia | Thalassemia | Leukemia |
|---|---|---|---|---|
| 1 - 6 | 0 | 0 | 3 | 9 |
| 6 - 12 | 0 | 0 | 3 | 2 |
| 12 - 20 | 1 | 0 | 4 | 0 |
| 20 - 40 | 1 | 4 | 35 | 3 |
| 40 - 60 | 1 | 5 | 14 | 3 |
| > 60 | 1 | 1 | 8 | 3 |
| Total | 4 | 10 | 67 | 20 |
5. Discussion
Approximately 5 - 10% of HTLV-I-infected individuals develop either ATL or HAM/TSP. Some evidences have shown that it has also been associated with other diseases such as cutaneous T cell lymphoma (CTCL), HTLV-I-associated arthropathy (HAAP), Graves’ disease, uveitis, polymyositis, chronic respiratory diseases, lymphadenitis, and dermatitis (20). On the other hand, HTLV-Ι and HTLV-II infections are usually chronic and untreatable diseases; so, adequate standards of diagnosis, prevention, care and support, as well as surveillance should be provided (21). HTLV-Ι infection is endemic in certain parts of the world (22) as well as in a northern city of Iran, Mashhad (14, 16). In the present study, we tried to determine the prevalence of HTLV-I and HTLV-II infections in patients with hematological disorders in Isfahan province, Iran. Although there is no defined treatment for patients infected with HTLV-Ι and HTLV-II, the accurate knowledge of the prevalence rates in different populations may be helpful in establishing prophylactic measures to reduce the rates of viral transmission from infected individuals. HTLV-Ι and HTLV-II are cell associated and spread in cells after blood transfusion, sexual intercourse, or breastfeeding.
Patients with hematological disorders who need repeated blood transfusion are among the most high-risk groups for this infection (23, 24). HTLV-Ι and HTLV-II, related to thalassemia and hemophilia by blood transfusion, have been reported in previous publications. In Iran, also, there is some evidence suggesting the relatively high prevalence of the virus in these patients. Farias de Carvalho et al. found a seroprevalence of 28.9% among patients with T-cell lymphoid malignancies in Brazil (25). Adedayo and Shehu found a 38.6% HTLV-Ι seropositivity in all hematological malignancies in India (12). Miyagi et al. found an HTLV-1 prevalence of 26.1% in 88 cases of non-Hodgkin’s lymphoma in Japan (26). The prevalence rate of HTLV-Ι and HTLV-II infections was 18% in southern Chile in patients with malignant hematological diseases, and 27% in patients with chronic lymphoproliferative disorders, as Barrientos et al. reported (27). Monavari et al. reported that the prevalence of HTLV-Ι among patients with malignant hematological diseases in Tehran was 12% (28).
The information about HTLV-Ι and HTLV-II prevalence in different populations and patients is crucial, because it may be useful in establishing prophylactic measures to decrease the rates of viral transmissions from infected individuals. Whereas the gold standard method for the diagnosis of HTLV infection is the detection of HTLV genome in specimens of patients, it seems that the prevalence of HTLV-Ι infection in our study population was 0.99%. PCR is an extremely sensitive technique, but sometimes has some limitations which lead to unexpected results. On the other hand, the presence of antibodies does not affect the results and the few copy number of the target sequence will appear in PCR product. Cross-contamination is the main limitation in PCR. Negative control was the indicator of cross-contamination and the PCR product of negative control did not show any expected amplicon length.
In the present study, we could not find any association between gender, age and occupation and HTLV-I. In conclusion, our study showed that the prevalence of HTLV-I infection in patients with thalassemia in Isfahan was 1.49%, but none of the samples contained the genome of HTLV-II. Therefore, it is necessary to carry out a large epidemiological study in this part of Iran. Although the prevalence of HTLV-I and HTLV-II infection among patients with hematological disorders in Isfahan province compared to other regions of Iran is not too high, HTLV screening should be performed prior to blood transfusion to decline the risk of virus transmission in these patients. Patients with HTLV-Ι and HTLV-II infection may acquire this infection via blood transfusion, despite all of the precautions for screening the blood supply. Therefore, the present study suggests that serious consideration must be given to prevent HTLV-I and HTLV-II infection via transfusion in hematological disorders. Routine serological screening for HTLV-I and HTLV-II antibody and detection of HTLV-Ι and HTLV-II genome in blood donors is indicated to permit the deferral of blood product donations by asymptomatic HTLV-Ι and HTLV-II carriers.