1. Background
Human Immunodeficiency virus (HIV) can infect and eliminate helper T-cells (CD4). Therefore, the ability of immune system to kill the germs such as bacteria, fungi, and viruses will be reduced due to elimination of these types of cells. The decreasing CD4 cell counts increase the susceptibility to opportunistic diseases. Consequently, HIV can cause the emergence of many opportunistic infectious diseases such as Tuberculosis (TB) and Pneumocystis Pneumonia (PCP), which are the most important and dangerous agents of pneumonia in immunosuppressed patients, especially those who are HIV positive (1). The prevalence of such diseases depends on geographical region and the CD4 cell count in the patients infected with HIV. While PCP was the most important and common reason of mortality in patients with HIV in the USA and European countries, before employing Highly Active Anti Retrovirus Therapy (HAART) and co-trimoxazole as prophylaxis against Pneumocystis jirovecii (2), Tuberculosis was the most prevalent infectious disease in the African regions (3, 4). Many reports are published on co-infection of more than one microorganism in most of the internal organs. However, there are few reports on the co-infection of an internal organ such as lung with two microorganisms (5-7).
2. Objectives
The current study aimed to evaluate the rate of co-infection with two opportunistic agents as P. jirovecii and Mycobacterium tuberculosis among the Iranian patients infected with HIV.
3. Patients and Methods
3.1. Patients
In the current retrospective study, forty-five pulmonary samples (39 sputum, four induced sputum, one bronchoalveolar lavage fluid, and one tracheal aspiration) were collected from 30 patients with HIV and positive results for M. bacilli in direct smear microscopy. The cases were selected among 126 patients with HIV referred to two referral centers, Iranian HIV/AIDS Research center (Imam Khomeini Hospital) and Mycobacteriology Research Center (Masieh Daneshvari Hospital) from August 2010 to March 2011. All of the patients showed at least one symptom of pneumonia including fever, cough with or without sputum, abnormal signs in their chest radiography, or computed tomography (CT) scan and pneumothorax. They were hospitalized because of pneumonia. Their CD4 cell count of the cases was under 200 cell/mm3. HIV was diagnosed by ELISA test and confirmed through western blot. Data regarding HIV status and the result of direct smear microscopy of acid-fast bacilli were extracted from medical records of the patients. M. tuberculosis complex was diagnosed and differentiated from M. avium-intracellular complex by IS6110-Polymerase Chain Reaction (PCR), as previously described (8).
3.2. DNA Extraction
Pulmonary samples of the patients were decontaminated and neutralized by 4% NaOH and 1% HCl, respectively. DNA was extracted using QIAamp DNA MiniKit (Qiagen, Germany) according to the instructions of manufacturer. Briefly, 20 µL of proteinase K and 200 µL of AL buffer were added to 200 µL of homogenized sample and incubated at 56°C for 10 to 20 minutes. After adding 200 µL of absolute ethanol to the solution, it was transferred to a spin column provided by QIAamp DNA MiniKit. Columns were washed twice with washing solution. Washing solution was discarded after centrifugation at 8000 rpm. Finally, 100 µL of the elution buffer (EL) or distilled water was added to the spin column and incubated at room temperature for one minute. DNA was collected in a 1.5 mm microtube by centrifugation at 8000 rpm. Extracted DNA could be directly used for PCR or stored at -20°C for later experiments.
3.3. PCR Test
A 260 base pair fragment of mt LSU rRNA gene of P. jirovecii was amplified by nested PCR. For the first round of PCR, two outer primers: PAZ102E:5’-GATGGCTGTTTCCAAGCCCA-3’ and PAZ102H:5’-GTGTACGTTGCAAAGTACTC-3’, and for the second step of PCR, two inner oligonucleotides: PAZ102-X: 5’-GTGAAATACAAATCGGACTAGG-3’ and PAZ102-Y: 5’-TCACTTAATATTAATTGGGGAGC-3’ were used as previously described (9-11). For the first round of PCR, 50 μg of the extracted DNA was added to a 50 µL PCR mix reaction containing: 10 mM Tris-HCl, 3 mM MgCl2, 0.4 mM of each dNTP, 10 pmol of specific primers for the first round of PCR plus 1 unit of Hot starTaq® DNA polymerase (Qiagene, Germany). The thermal condition was provided by a thermo cycler machine (Astec, Japan) as follows: initial denaturation at 95˚C for five minutes, followed by 35 cycles: 95˚C for 30 seconds, 55˚C for 40 seconds, 72 ˚C for 60 seconds, and final extension at 72˚C for 10 minutes. The second round of PCR was performed in the same condition except that instead of the target DNA, 5 µL of amplicon from the first round of PCR was added to a mix containing inner specific primer.
3.4. Detection
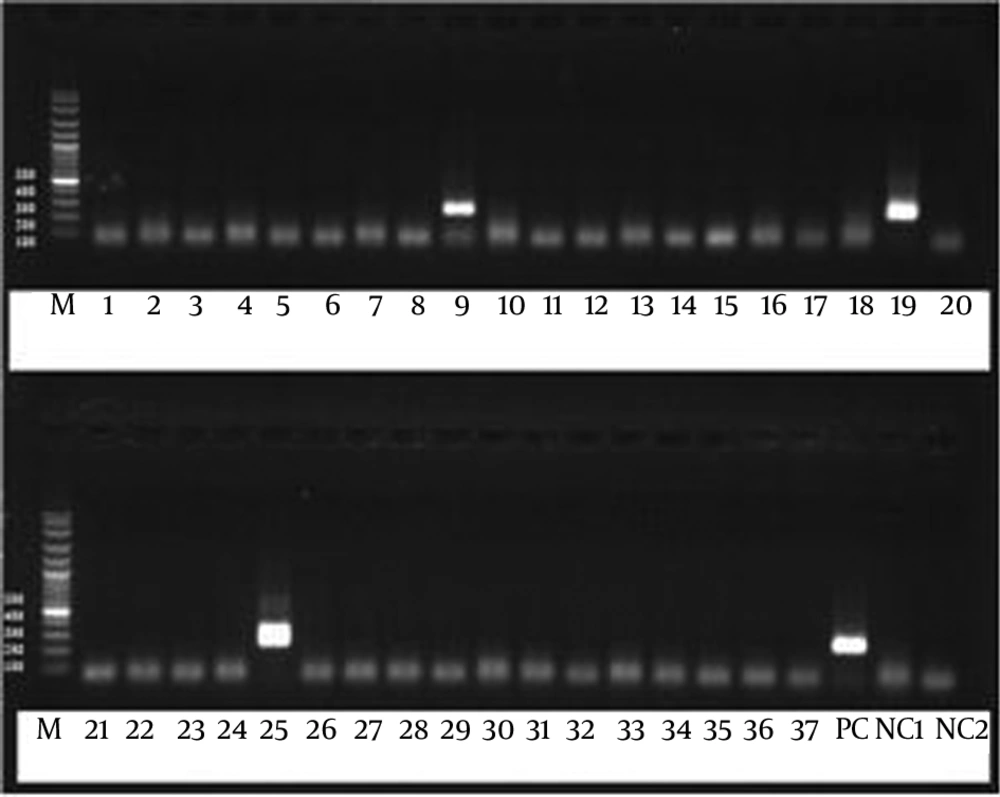
To detect the desired bands, 260 bp of mt LSU rRNA gene, the PCR products were run on 1.5% agarose gel containing ethidium bromide and visualized under ultra violet (Figure 1). To prevent cross contamination, the steps of procedure were performed in separate completely equipped rooms. Distilled water was used instead of the target DNA as negative control in each step of the procedure. The DNA extracted from sputum of a patient with confirmed Pneumocystis pneumonia was used as positive control.
4. Results
All of the patients with HIV introduced to this project were male with an average age of 32.95 ± 7.15 year (ranging from 18 to 69). The mean of CD4 cell counts was 109.25 cell/mm3. Based on the protocol provided by the Ministry of Health and Medical Education of Iran, all of the patients were under TB treatment and they received ART (Anti Retroviral Therapy) and co-trimoxazol as prophylaxis against Pneumocystis pneumonia. The study results showed that the rate of tuberculosis among the Iranian patients with HIV was 42% (30 out of 126 cases) and three out of 30 cases with HIV (10%) were co-infected with M. tuberculosis and P. jirovecii (Figure 1). No other infectious agents were detected in them. Notably, the CD4 cell counts of the patients co-infected with the mentioned microorganisms was under 50 cell/mm3.
5. Discussion
Opportunistic bacterial and fungal diseases such as tuberculosis and Pneumocystis pneumonia may cause fetal illness in patients with HIV (1). Among the opportunistic diseases, Pneumocystis pneumonia is more dangerous for immunosupprressed patients, especially in those infected with HIV. There are many reports on the co-infection of P. jirovecii with Cytomegalovirus, Cryptococcus spp., Aspergillus spp., Herpes simplex, Mycobacteriumavium-intracellular complex in patients infected with HIV (12-14). However, there are few studies on the co-infection of an internal organ such as lung with two opportunistic microorganisms, P. jirovecii and M. tuberculosis, in these patients (5-7, 15). To the authors’ best knowledge, the current study was the first on co-infection of Pneumocystis pneumonia and tuberculosis among the Iranian patients infected with HIV. Results of the current retrospective study showed the rate of co-infection with these two opportunistic microorganisms in Iranian patients with HIV as 10%, which was similar to those of the African study (9.9%) (16).
Before introducing ART and co-trimoxazole as a powerful prophylaxis against PCP in patients with HIV, Pneumocystis pneumonia was one of the most prevalent and life-threatening diseases in the developed countries (17). On the other hand, the prevalence of tuberculosis was higher than PCP in South Africa and it was the most important and prevalent infectious disease in patients with HIV in this region (3, 4, 17). Results of the current study indicated that the prevalence of tuberculosis in Iranian patients with HIV was 42%. The previous studies revealed that the rate of PCP in these patients was 12.3% (18). Another study conducted in Ethiopia was concordant with the current study. Although, the rate of co-infection with these two microorganisms in Ethiopia was relatively higher (13.5% of patients with HIV also infected with tuberculosis), it should be noted that, based on WHO reports the prevalence of tuberculosis in Iranian patients with HIV on ART and Co-trimoxazole Preventive Therapy (CPT) was significantly lower than those of the Ethiopian patients (18-20). Logically, the probability of co-infection with two microorganisms of P. jirovecii and M. tuberculosis was higher in regions with a high rate of TB.
Based on the protocol provided by the Ministry of Health and Medical Education of Iran, all of the infected patients with HIV received ART, Co-trimoxazole as a prophylaxis against PCP and Toxoplasmosis, especially when their CD4 cell counts were under 200 cell/mm3. Additionally, isoniazid as prophylaxis against LTBI was applied to the patients especially when they had close contact with TB patients or their PPD test (Purified Protein Derivative) had shown indurations. It seemed that, if Iranian patients with HIV were not under ART therapy and prophylaxis against PCP and they did not received isoniazid and rifampicin as the standard regimen for the tuberculosis treatment, the rate of co-infection of Pneumocystis pneumonia and tuberculosis would be higher. In contrast, infected patients with HIV in South African did not get prophylaxis against P. jirovecii and specific treatment against tuberculosis prior to clinical manifestation. Therefore, it could be supposed that the prevalence of P. jirovecii in South Africa was low in comparison to Iran. It was confirmed by earlier studies that the prevalence of PCP in Iranian patients with HIV positive was reported significantly higher than in the African adult patients with HIV (17, 18, 21, 22).
Interestingly, the CD4 cell counts of the patients who co-infected with these two opportunistic microorganisms were reported less than 50 cell/mm3. It was confirmed that with the progress of HIV disease and worsening of the immune status, the probability of opportunistic and life-threatening diseases such as Pneumocystis pneumonia would be higher (23). In the early phases of HIV, the rate of opportunistic diseases with high frequency such as tuberculosis would be higher, but with disease progression and immune deterioration, greater incidence of PCP, which is a disease with lower frequency, would be observed. Based on a study, patient with HIV were infected with tuberculosis when their CD4 cell counts were decreased to 206 cell/mm3; whereas, the PCP occurred when their CD4 cell counts were 134 cell/mm3 (24). Although the number of patients who co-infected with these two microorganisms was low in the current study, the above rule was true.
The study was conducted in 30 cases out of 126 Iranian patients with HIV who their smear samples were positive in microscopy test for M. tuberculosis. The probability of PCP co-infection with tuberculosis among the patients of this category was high because tuberculosis was an endemic disease in Iran. With worsening the immune status patients with HIV, due to decrease of CD4 cell counts, the probability of Pneumocystis pneumonia occurrence will increase. The co-infection with these two microorganisms in patients with HIV was ignored most of the times, since the physicians were not aware of it. Therefore, the most rapid and sensitive tests to detect PCP should be applied and the patients should receive appropriate treatment.