1. Background
Microsporidiosis is considered as an opportunistic infection in immunodeficient patients such as patients with acquired immune deficiency syndrome (AIDS), transplant recipients, children, elderly, people with ophthalmic lenses, and passengers (1). The phylum microorganisms includes about 1200 species and 150 genera. Enterocytozoon bieneusi as well as Encephalitozoon species (E. hellem, E. intestinalis and E. cuniculi) are the most frequent causes of human microsporidiosis (2). The microorganisms are an obligate intracellular parasite that can infect a wide spectrum of invertebrate and vertebrate hosts (3, 4). The life cycle of this parasite consists of 3 separate stages: infectious stage, replicating or growing stage, schizogony and spore formation stage, or sporogony (5). Microsporidia can be the cause of the wide spectrum of symptoms such as keratoconjunctivitis, myositis, hepatitis, sinusitis, chronic diarrhea, disseminated infections (1), nausea, severe weight loss, and confusion (6).
It is reported that microsporidia is most likely transferred by water contaminated via the stool of an animal (7). Microsporidia have been detected in various animals and humans and it is indicator of the zoonotic potential, however, valid documents for the transmission from animals to humans are lacking (8). It is reported that some patients with ocular microsporidiosis were exposed to pet birds (9). Also, exposure to other animals might be a significant link in the epidemiology of zoonotic microsporidiosis (8, 10).
2. Objectives
The research in Iran, on the prevalence of the microorganisms, have been performed, including the prevalence investigations in various animals and pigeons (11, 12), in liver transplant recipient patients (13), and HIV+/AIDS patients (14); however, the current study is a novel research in wild rats. Although, the parasites are self - limited in immunocompetent people, it can be life - threatening in immunodeficient patients (3, 7). For this reason, it is due to the increasing prevalence of parasitic infections and immunodeficiency diseases as well as the transmission risk of microsporidia from animals to human; hence, the aim of this study was to evaluate the identification of E. bieneusi and Encephalitozoon spp. in wild rats of Ahvaz city, southwest of Iran.
3. Methods
3.1. Ethics Statement
All animal experimental procedures were approved by the Animal Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (CMRC code number 5883).
3.2. Sample Collection
Initially, 160 stool samples were collected from wild rats in different parts of Ahvaz city in 2012 - 2014. For this purpose, the Sherman trap was employed. In each sample, the trap was installed at sunset and collected before sunrise (15). The wild rats (Rattus norvegicus and R. rattus) were hunted in 5 points of Ahvaz city (South, North, East, West and Central). After entrapment, the rats were transferred to the Department of Parasitology, Ahvaz Jundishapur University of Medical Sciences. After killing of rats through euthanasia and collecting the stool samples, a part of the stool sample was used for the staining. The rest of the feces were mixed with twice the volume of potassium dichromate 2.5% and kept at 4 °C (16).
3.3. Samples Examination by Staining
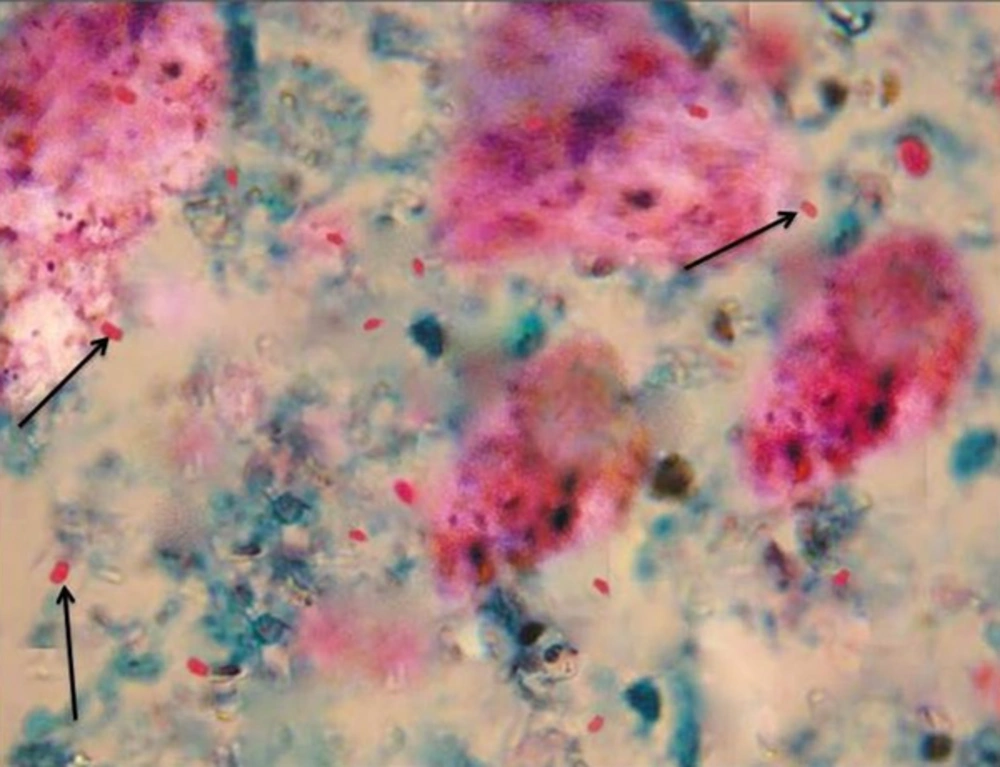
The stool samples were stained by the modified trichrome (weber). The slides were fixed with methanol and placed in trichrome color for 240 minutes. After decolorization with acid - alcohol, and washing with 95% ethanol, the slides were placed in absolute ethanol. To identify the spores, the slides were examined by an optical microscope at magnification of x100 together with immersion oil. The positive samples were identified by dorsal vacuoles in microsporidia spore (17).
3.4. Extraction of DNA
The DNA was extracted by DNA stool kits (Bioneer, South Korea) and the extracted DNA was stored at -20 °C. This kit consisted of spin column, where the column absorbed the parasite DNA. After washing it twice with special buffers, the purified DNA was obtained (17).
3.5. Molecular Detection
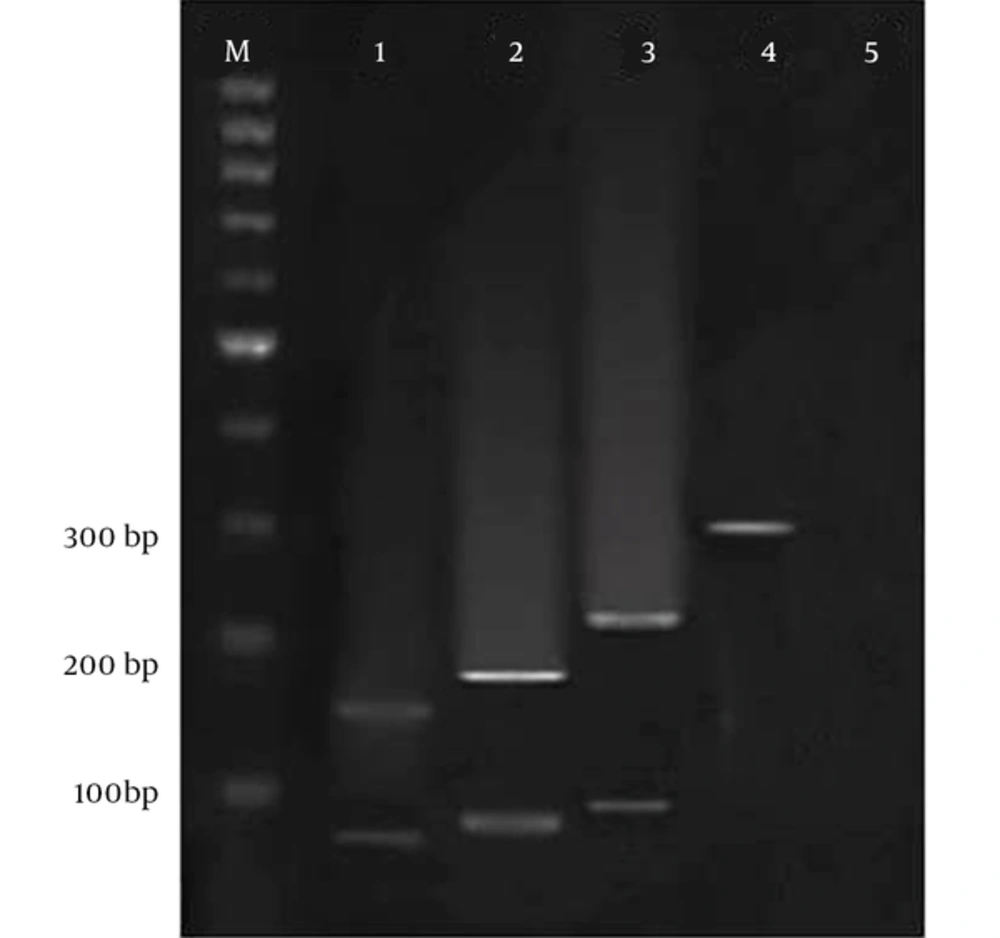
The extracted DNA was examined by the multiplex/nested PCR method, where the microsporidial genera of Enterocytozoon and Encephalitozoon were identified by this method. At this stage, we used specific primers that were designed by Katzwinkel et al. (18). These primers were designed based on small subunit ribosomal RNA (16S rRNA) gene where the primers were used for the identification of different species of microsporidia. The primers were purchased from the Bioneer Company and stored at -20 °C. Table 1 shows the primary and secondary primers for multiplex/nested PCR method. The amplified fragment length by the primers was 500 bp and 300 bp for the microsporidial genera of Enterocytozoon and Encephalitozoon, respectively. At first, the samples were examined with the primary and secondary primers by multiplex/nested PCR method. Then, for differentiating the species of Encephalitozoon, the multiplex/nested PCR products were explored by RFLP method using the restriction enzyme of Mnl1 (17).
| The Primary Primers | The Secondary Primers |
|---|---|
| MSP-1: TGAATGKGTCCCTGT | MSP-3: GGAATTCACACCGCCCGTC(A,G)(C,T) TAT |
| MSP-2A: TCACTCGCCGCTACT | MSP-4A: CCAAGCTTATGCTTAAGT(C,T)(A,C)AA(A,G)GGGT |
| MSP-2B: GTTCATTCGCACTACT | MSP-4B: CCAAGCTTATGCTTAAGTCCAGGGAG |
3.6. Sequencing
For genotyping, the positive samples of RFLP were sequenced by Bioneer Company (Daejeon, South Korea). Afterwards, the specified sequence was compared against the sequence of the registered isolates available in the GenBank library (NCBI) and homology between them was examined by BLAST software (17).
4. Results
4.1. Staining
Figure 1 demonstrates microsporidia spore in the feceal samples obtained from wild rats. Of 160, 26 specimens were suspected positive for the parasite spore by the staining.
4.2. Molecular Analysis
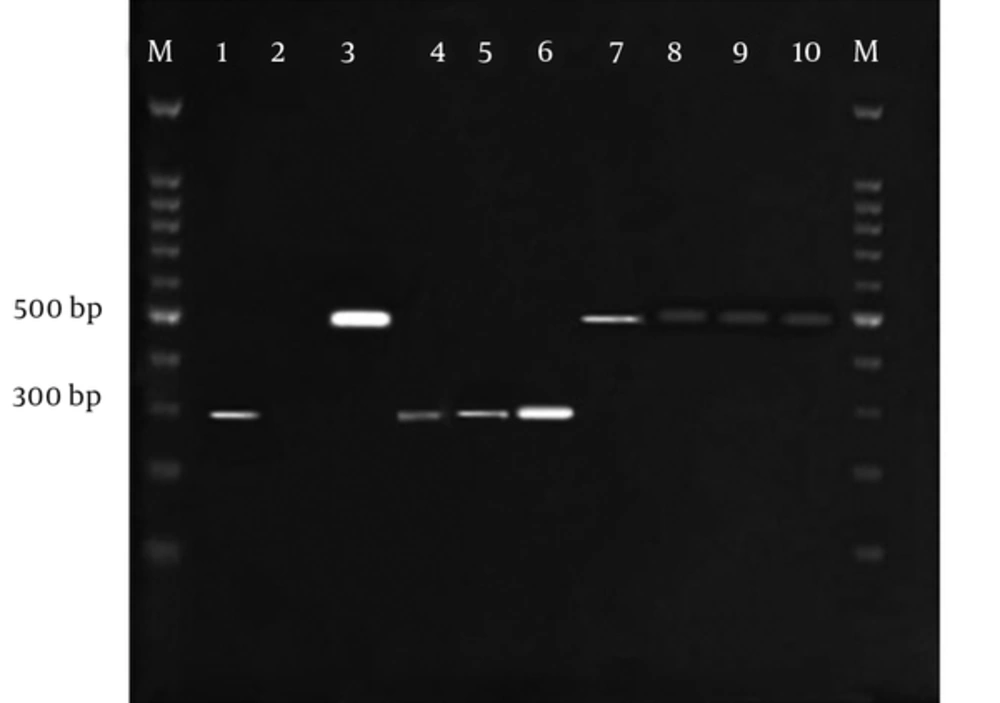
Figure 2 indicates electrophoresis of the SSU rRNA gene PCR products on Agarose gel 2% and Figure 3 shows electrophoresis of the RFLP products on agarose gel 3%. Table 2 demonstrates the findings of molecular diagnosis and genotyping of the stool samples of wild rats. Based on Table 2, 2 species of wild rats were R. rattus (14 samples) and R. norvegicus (146 samples). Of the 160 samples, 18 cases were positive for the microorganism by the Multiplex/nested-PCR method. Therefore, 14 and 4 samples were detected as E. bieneusi and Encephalitozoon spp, respectively. Encephalitozoon species consisted of 4 E. intestinalis, only.
The electrophoresis of the SSU rrna gene PCR products on agarose gel 2%, the amplified fragment length was 500 bp and 300 bp for gender of Enterocytozoon and Encephalitozoon, respectively. M: 100bp DNA marker, sample 1: Positive control for Encephalitozoon spp., 2: Negative control, 3: Positive control for E. Bineusi, 4 - 6: Positive samples for Encephalitozoon spp., 7 - 10: Positive samples for E. Bineusi.
The electrophoresis of the RFLP products on agarose gel 3%, for differentiating the species of Encephalitozoon, the multiplex/nested PCR products were explored by RFLP method using the restriction enzyme of Mnl1, M: 100 bp DNA Marker, 1: Encephalitozoonintestinalis (160 and 60 bp), 2: E. hellem (180 and 80 bp), 3: E. Cuniculli (210 and 90 bp), 4: Encephalitozoon spp. Without enzyme.
| Samples | Number | Positive | E. bieneusi (Genotype) | Encephalitozoon spp. | E. intestinalis | E. hellem | E. cuniculi |
|---|---|---|---|---|---|---|---|
| Rattus norvegicus | 146 | 17 | 11 (D) 2 (M) | 4 | 4 | 0 | 0 |
| R. rattus | 14 | 1 | 1 (D) | 0 | 0 | 0 | 0 |
| Total | 160 | 18 | 14 | 4 | 4 | 0 | 0 |
4.3. Sequencing and Genotyping
Aaccording to Table 2, of the 14 positive samples of E. bieneusi, 12 and 2 cases were identified as genotype D and M, respectively. Only a case of microsporidia (E. bieneusi with genotype D) was observed in the R. rattus species and 17 cases were detected in the R. norvegicus species. The data of nucleotide sequences indicated in the article are available in GenBank at accession numbers KY972247 - KY972264.
5. Discussion
Infectious diseases can threaten the public health (19). The infections are one of the major causes of mortality in patients with impaired immune systems. Among them, microsporidiosis is considered as an opportunistic infection in immunodeficient patients (1). In the past, this view was that E. bieneusi, E. intestinalis, and E. hellem can be seen only in humans, however, afterwards, E. bieneusi and E. intestinalis were also detected in a wide range of domestic and wild animals (20). On the other hand, E. hellem was identified in the birds (21). There are also E. cuniculi in various animals. These data indicated that the 4 main species infecting humans have the zoonotic nature (22). Accordingly in this study, we investigated microsporidiosis in wild rats in 5 regions of Ahvaz (north, south, east, west and central). Due to the direct and indirect relationship with humans, rats were selected.
The specie of E. bieneusi is the most frequent specie isolated from immunodeficient patients, animals (8), and even immunocompetent people (14, 23). In the current study, also, these microorganisms had the highest prevalence in wild rats. The results of this study showed that of 160, 14 samples were positive for E. bieneusi and of the 14 samples, 12 cases were detected as genotype D. Consistent with our findings, genotype D has been reported as the most frequent genotype in most studies (24). In Thailand, Leelayoova et al., in 2006, demonstrated that genotype D with 36.4% had the highest prevalence of genotype in AIDS patients and the phylogenetic analysis suggests the zoonotic potential of the parasite (25). Our findings, besides other studies, indicate that genotype D is in most of the immunodeficient patients and animals that could be associated with the widespread potential of the genotype. Also, E. bieneusi has been identified in chickens, grey parrots, pigeons, cockatiels, lovebirds, finches, and falcons (26-29).
According to the results of the study, of 14 E. bieneusi species, 2 cases were identified as genotype M. Also, previous studies showed that genotype M was observed in animals (30). On the other hand, our findings show that 4 cases were detected as Encephalitozoon species, which included 4 E. intestinalis. Encephalitozoon intestinalis is the 2nd most frequent microsporidial parasite of human (8) and these results are consistent with our findings. Also, in several investigations, the microorganism was identified in immunodeficient patients (8). These studies along with our study show that there is the transmission risk of certain genotypes to new host; this genotype was not seen in the host previously. In contrast to the studies and our study, in 2009, Kasickova et al., reported that microsporidial DNA was detected in 115 fecal samples (40.1%) but no E. intestinalis positive samples were detected (31).
The current study was a novel research in wild rats and significant in terms of health, due to the fact that the microsporidial species were isolated from wild rats of Ahvaz city, southwest of Iran. Due to the direct and indirect relationship of rats with humans, these animals are an important source of pollution. Therefore, for the design of proper precautions for microsporidiosis, it is important that the high - risk people, such as immunodeficient patients, should be receiving the information about the risk of direct and indirect contact with these animals. On the other hand, it is recommended to investigators to evaluate the various hosts such as the wild animals or domestic animals as well as the role of the animals and the mechanisms of the transmission in high - risk people. Hence, it is essential to evaluate the wide spectrum of epidemiological and molecular investigations on the hosts.
6. Conclusion
In conclusion, the findings revealed a relatively high prevalence of microsporidia infection in wild rats of the city and these animals can be a source of microsporidiosis. Due to the zoonotic potential of the microorganisms, high - risk individuals should be receiving the information about the risk of direct and indirect contact with the infected animals.