1. Background
Bacterial urinary tract infection (UTI) is the most common kind of infection affecting the urinary tract and causing inflammation of bladder and kidneys. Urine is a favorable medium for growth of bacteria due to its enriched chemical composition (1-3). UTI is the third most common cause of admission to hospitals in India. It has been estimated that about 6 million patients per year are visited worldwide for UTI out of which around 30,000 are treated in the wards. (4). Particularly, those sub-populations who are at increased risk of UTI include infants, pregnant women, and elderly of both sexes, as well as those with spinal cord injuries, indwelling catheters, diabetes, multiple sclerosis, immunodeficiency, and underlying urological abnormalities.
Majority of women are recurrently infected within one year (5). Compared to males, young sexually active females and teenage students girls are mostly infected by UTI (10.57% higher). In this group, most prominent pathogens are Escherichia coli (32.8%), Klebsiella pneumoniae (22.4%), and Staphylococcus aureus (15.1%) (6). E. coli cause 75 – 90% of uncomplicated UTIs (7) whereas S. saprophyticus frequently causes UTI in younger women at estimated rate of 5 – 15%(8). Golechha and Solanki (9) performed a bacteriological study on preoperative urine samples of 100 urolithiasis cases in India. E.coli was encountered as the commonest pathogen recovered from pre-operative urine and stone cultures (32.25% and 21.73%, respectively) followed by Pseudomonas aeruginosa (22.58% and 17.39%, respectively). Enterococcus and other Gram negative rods other than E.coli have also been implicated in some cases (10).
UTI treatment incurs a considerable cost, both directly and indirectly, on the health care system. In the United States of America, approximately 5% of all patients acquired infections after admission to hospital with an estimated cost of $1.6 million annually (11-13). The predominant urinary tract pathogens are E.coli (93%); other minor groups include Enterococcus sp., hemolytic Streptococci sp., S. aureus, and Pseudomonas sp.
Antimicrobial susceptibility profile in respect to causative microbes may significantly reduce morbidity and mortality, cost of treatment, and duration of hospitalization if duly provided to medical practitioners and clinicians in a rapid and timely fashion (14).
2. Objectives
The West Medinipur is a habitat of diverse group of populations including primitive tribal like Lodha, Kheria / Sabar, Munda, Santal, Kohl, Oraon, Mahali, and Bhumij. Because of poor health care system, most of clinicians prescribe antibiotics to treat UTI blindly, resulting in failure of treatment in many cases due to occurrence of bacterial drug resistance.. The major objectives of this study include isolation and identification of predominant UTI causing bacteria in different groups of tribal people and observation of the current sensitivity trend of most common commercial drugs against predominant pathogens. This may also highlight the prevalence of pathogens in tribal population of different age and sex.
3. Patients and Methods
3.1. Study Design
Urine samples of clinically suspected outpatients with UTI as well as hospitalized patients were collected. A detailed history was taken and complete clinical examination was carried out for each case of UTI. Some samples were collected from clean catch midstream; in neonatal cases the samples were collected through suprapubic approach and in children (less than 3 years) by sterile urine bags (any colony count of bacterial growth was significant). It was noticed to discontinue all antibiotics 72 hr prior to urine collection for culture and sensitivity. Urine samples were delivered to the laboratory within 1hr and processed within 24 hr from collection. Each and every sample was observed under microscope and examined for the presence of puss cells, RBCs, epithelial cells, casts, and crystals. A total number of 4,416 early morning midstream urine samples of patients from different areas were collected from June 2006 to October 2007.
3.2. Isolation and Identification
A standard loop technique was used to place 0.01 mL of urine for inoculation on Blood agar, MacConkey’s agar, and cystine lactose electrolyte deficient (CLED) agar, and incubated at 37oC for 24 hr and extended up to 48 hr in cases of negative growth. At any multiple growths, the culture was repeated before acceptance of outcomes. All positive samples were rechecked by collecting second urine samples to rule out contaminations. The number of colonies was counted to quantify organisms. Diagnosis of urinary tract infection for a single pathogen was defined based on significant colony count of ≥ 105 CFU/mL for Gram negative and ≥ 104 CFU/mL for Gram positive bacteria. The organisms were identified through monitoring general biochemical tests such as catalase, oxidase, Triple Sugar Iron agar (TSI), citrate utilization (Simmon’s citrates medium), urease (Christensen’s Urea Agar), indole, motility, H2S production (Sulphide Indole Motility Medium), esculin hydrolysis (Bile esculin agar), and sugar fermentation tests. All culture media were provided by Himedia Laboratories Pvt. Ltd., India.
3.3. Antimicrobial Susceptibility of Most Common Bacteria of UTI
Antibiotic susceptibility testing against the most common causative bacteria was performed according to Kirby Bauer’s method (12). The antibiotic discs (Himedia, India) (each 6.3 mm diameter) used were Amoxicillin with clavulanic acid (30mcg), Ceftazidime(30mcg), Cefoperazone (75mcg), Cefotaxime (30mcg), Cifixime (5mcg), Ceftriaxone (30mcg), Tobramycin (10mcg), Gentamycin (10mcg), Netilmycin (30mcg), Amikacin (30mcg), Co-trimoxazole (25mcg), Gatifloxacin (5mcg), Norfloxacin (10mcg), Levofloxacin (5cg), Ofloxacin (5mcg), and Meropenem (10mcg). The zone of inhibition of each antibiotics against the causative bacteria were compared using standard CLSI protocol (15).
4. Results
To find out predominant causative organism of UTI in our district, 4,416 urine samples (both community and hospital acquired) were cultured from different sexes and ages of tribal population.
In 1,596 out of 4,416 samples, bacterial growth was observed as a cause of significant and insignificant urinary tract infection. Among them 1,190 samples showed significant bacteriuria, 383 samples showed insignificant bacteriuria, and 23 samples showed colony count between 104–105 CFU/mL of mixed growth which was considered as contaminated samples (16-18). Urine samples with negative growth were marked in 2,820 cases.
Generally, we had identified seven bacteria sp. mainly E.coli, Klebsiella sp., P. aeruginosa, Proteus sp., S. aureus and coagulase negative streptococci (CONS), Enterococcus sp., and others Gram negative spp which consists of Alkaligenes feacalis, Acinetobacter bawmani, Providentia sp., and some Enterobacter spp.
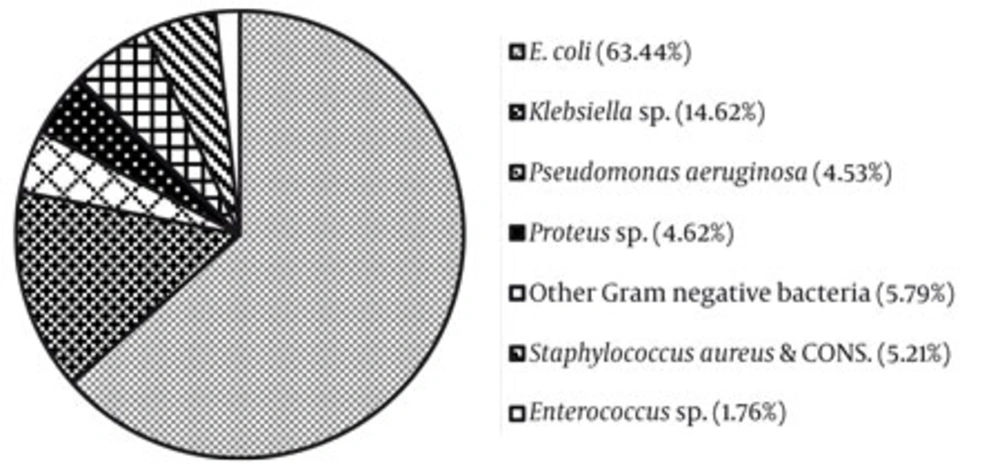
In our study it was observed that E. coli was the most common causative organism for UTI in both community and hospital acquired infection (63.44%). Klebsiella sp. was the second most common isolated organisms (14.62%) in all ages of this population. The infection rate of P. aeruginosa and Proteus sp (both P. vulgaris and P. mirabilis) were 4.53% and 4.62%, respectively in respect to total number of positive growth samples.
Gram positive bacteria including Staphylococcus sp. and Enterococcus sp. were responsible for UTI in both genders. In our West Medinipur zone from tribal community, the rate of positive samples for S. aureus & CONS, and Enterococcus sp were 5.21% and 1.76%, respectively. Infection rate by other unidentified species was 5.79% (Figure 1).
In respect of sex variation, isolated bacteria were sex-dependent. The percentages of Pseudomonas sp. and Proteus sp. were slightly higher in male patients, whereas females were infected more by E.coli and Klebsiella sp. (Figure 2).
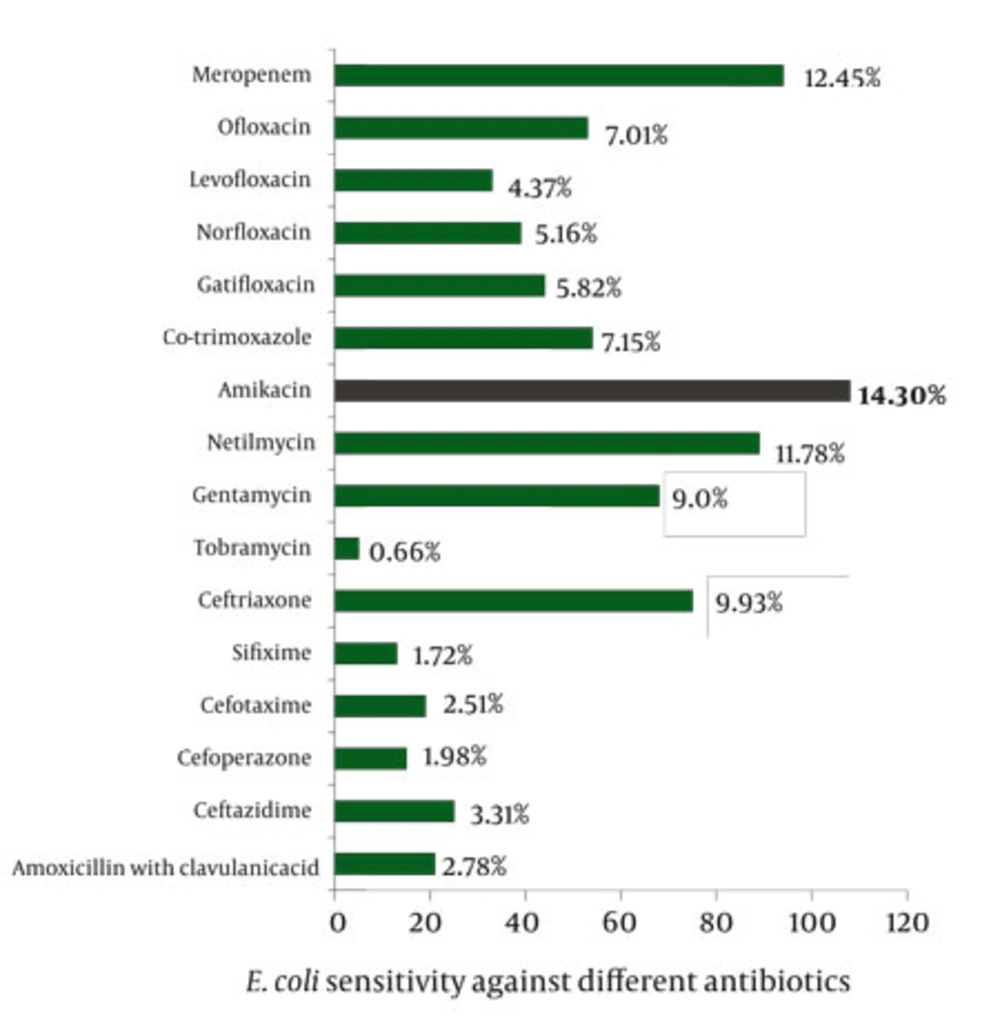
Antibiotic susceptibility tests were performed for common isolated pathogen E.coli. 755 out of 1,190 positive growth, showed positive growth of E. coli. All of these isolates were processed individually to pass sensitivity test against 16 different antibiotics. Figure 3 revealed antibiotic susceptibility pattern of different isolated E.coli, in which Amikacin (14.30%) was the most common effective drug followed by Meropenem (12.45%). One aminoglycoside, Tobramycin, was the least effective drug on E.coli in UTI.
5. Discussion
Asymptomatic bacteriuria is the major cause of UTI because under favorable conditions colonized bacteria in urinary tract may ascend towards the bladder and cause cystitis which is usually associated with the classic symptoms of UTI (i.e., pain, frequency, and urgency). If UTI remains untreated, it can proceed via ureters, to the kidneys that may cause pyelonephritis which may lead to irreversible kidney damage, renal failure, and death (16, 17).
The tribal people have different culture, custom, and life style and most of them are dependent upon natural medicine. Due to poor economic condition, they are not able to avail the facility of modern medicinal system. This is probably the first study in this group of people to understand prevalent organism for UTI and related antibiotic sensitivity. In this study, we found that percentage of urinary tract infection with significant bacteriuria was 74.56% among 1,596 positive growth samples with suspected fever with or without UTI symptoms, randomly. A study was conducted among 1,000 pregnant women with asymptomatic UTI from National Medical College; Calcutta that revealed the prevalence of bacteriuria was 10.2% (16). In this study a comparable different pattern of bacterial prevalence was found among UTI infected tribal people such as E.coli (63.44%), Klebsiella sp. (14.62%), P. aeruginosa (4.53%), Proteus sp. (4.62%), S. aureus and CONS (5.21%), Enterococcus sp. (1.76%) and other Gram negative spp. including Alkaligenes feacalis, A. bawmani, Providentia sp., and some Enterobacter (1.76%).
E.coli was the commonest bacteria (63.44%) in UTI patients, but with a different rate obtained from other populations in U.S.A. study (75.5% - 87.0%) (19, 20), where the number are 68.69% and 83.0% as seen by Rayan et al. in general population in India (21). On the contrary, in the report of Nabeela et al., Klebsiella sp. and Proteus sp. were responsible for 16% and 11% of all urinary tract infections, respectively (22). This variation may be attributed to different life style, poor healthcare system, lack of education, and inadequate availability of water, and also may be due to geographical variations.
However, it should be pointed out that other investigators have observed E. coli as the common bacterium in significant bacteriuria. In our study same findings were obtained in tribal people of West Medinipur zone and enriched the fact that females are more susceptible to UTI than males and that the most effective drug (effective on isolated E.coli) is Amikacin (from amino-glycosides group) for this tribal people. Therefore, culture and antimicrobial drug sensitivity testing are prepared for surveillance purposes to guide clinicians on proper management and prevent empirical treatment of tribal population with both asymptomatic & symptomatic bacteriuria.
Our study will be a common and convenient database for medical practitioners for treatment of UTI patients in tribal population of Paschim Medinipur, West Bengal, India.