1. Background
Enteroviruses are small, non-enveloped viruses with icosahedral symmetry. They are members of Picornaviridae family that cause wide spectrum of diseases such as respiratory infection, gastroenteritis, sepsis, myocarditis, and meningitis (1). They have a single stranded positive sense RNA as genome with only one ORF (open reading frame). The genome consists of three parts as P1, P2, and P3. P1 encodes capsid protein and two other parts encode non-structural proteins such as proteases.
Enteroviruses are divided in four sub-groups including human enterovirus A (HEV A), human enterovirus B (HEV B), human enterovirus C (HEV C), and human enterovirus D (HEV D) (2, 3). Aseptic meningitis is one of the most important diseases related to enteroviruses that are responsible for about 80 to 90 percent of viral meningitis over the world. The disease is recognized by symptoms such as fever, headache, neck stiffness, and photophobia that mostly involve children and young adults. In children and newborns, infection can spread through the body and cause some serious diseases like hepatitis and myocarditis. These viruses are transmitted via oral-fecal route and mostly seen in fall and summer at temperate areas (4).
Echovirus 30 as a member of enteroviruses was reported from some countries such as Kuwait, Turkey, Brazil, and Korea (2, 5-7). Cell culture was the gold standard and classical diagnostic method for detection and isolation of enteroviruses but as this method is very time consuming and needs much facilities, after the advent of molecular techniques such as PCR most studies are performed based on these techniques (8).
2. Objectives
In this survey, 34 enterovirus positive cerebrospinal fluid (CSF) samples were collected during one year and investigated by RT-PCR (reverse transcription polymerase chain reaction), cell culture, and sequencing to determine relative frequency of echovirus 30 as an important agent of aseptic meningitis.
3. Materials and Methods
3.1. Patients
In this study, we investigated 34 CSF samples collected between May 2010 and May 2011 from 57suspected patients less than 14 years old (average: 1.58 ± 2.25 years old) with aseptic meningitis who were referred to Aboozar hospital, Ahvaz, Iran. The sample collection was performed with patient’s consent. These 34 cases suffered from enterovirus meningitis already approved by RT-PCR test in previous study (9). Thirty four patients with symptoms of aseptic meningitis, negative bacterial culture, WBC count > 5×106/mm3 , and positive RT-PCR test for enteroviruses with pan enterovirus primers were entered in the study. Most of the cases were under 2 years old (about 80%); also a 2-day-old patient who suffered from heart attack was recognized among the patients. Most cases occurred in winter and fall respectively with no significance relationship between spring, winter, and fall (P > 0.05); these information were already mentioned in previous study (9). Clinical symptoms and laboratory characteristics of patient’s CSF were recorded and shown in Table 1 and Table 2, respectively.
3.2. Virus Extraction and cDNA Synthesis
Viral RNA was extracted from CSF samples with a high pure viral nucleic acid kit (Roche Diagnostics, Indianapolis, USA) according to manufacturer’s instruction. cDNA was synthesized using a cDNA synthesize kit (Vivantis, Malaysia) in two steps according to manufacturer’s instruction. In the first step, a mixture of 1µL random primer (2.5 ng/µL), 1µL dNTP (0.5 mM), 1µL mgcl2 (2.5 mM), 6µL extracted viral RNA, and 1µL of diethylpyrocarbonate (DEPC) water was prepared in a 10µL volume and incubated at 65ºC for 5 minutes and chilled on ice for 2 minutes, respectively. In the second step, a mixture of 2µL 10X buffer M-MuLV, 0.5µL M-MuLV reverse transcriptase (100U/µL), and 0.5µL RNase inhibitor (20U/µL ) in 7µL of DEPC water was prepared and added to the first mixture, mixed, and then incubated in 42ºC for 60 minutes. The reactions were incubated at 85ºC for 5 minutes to terminate the reaction.
3.3. RT-PCR Amplification for Echo 30 Detection
CSF samples of 34 patients with enterovirus meningitis were investigated for echovirus 30 by RT-PCR. The specific primers for echovirus 30 consisted of 120 S (GACCCIGARIRIGCIYTNAA) ( VP1, 4-23 ) and 47 A (TKIACRTGICKIGTYTGCAT) ( VP1, 143-162 ) with the PCR product of 158 bp (9). PCR amplification were prepared in a final volume of 25µL containing 5µL of cDNA, 2.5µL 10x viBuffer, 0.5µL dNTP (10 mM), 0.125µL taq polymerase (0.625 unit) , 1.5 mM MgCl2, 0.75µL from each primer (0.3 µM), and water up to 25µL. The cycling was programmed as:; incubation at 94ºC for 5 minutes and 35 cycles with denaturation at 94ºC for 30s, annealing at 52ºC for 30s, extension at 72ºC for 30s, and final extension at 72ºC for 5 minutes . 10µL of PCR product was mixed with 6x loading dye and loaded onto 2% w/v agarose gel. After the electrophoresis (100 volts for 50 minutes, 0.5x TBE buffer) the gel was stained in ethidium bromide (0.5 µg/ mL), the amplicon was visualized, and its size was determined using UV trans-illuminator (Lambert, France) to observe 158 bp band.
3.4. Virus Isolation
We used cell culture to propagate the virus and increase its titer. Culture also was used to distinct between virion and defective particles. Achieving this goal, we used RD cells (rhabdomyosarcoma) isolated from a kind of malignant skeletal muscle cell. After preparation of culture tubes with 70% to 80% confluent RD cells, about 200µL from each of 34 samples was added to each tube, incubated the tubes in an incubator with 37ºC temperature and 5% CO2 for one hour to attach viruses to cell receptors, and shaken the tubes gently every 15 minutes for even distribution of samples on tubes surfaces. After 1 hour, tubes were discharged, about 1 mL maintenance MEM media (MEM media with 2% calf serum) was added, and kept in 37ºC incubator with 5% CO2. Tubes were monitored daily for 7 days to observe cytopathic effect (detached cells, round cells ) (9).
3.5. Virus Extraction from the Culture
The enterovirus RNA was extracted from supernatant of cultures with Tripure reagent (Roche Diagnostics, Indianapolis, USA) and chloroform. Isopropanol and ethanol were used to precipitate and sediment RNA which was hydrated with 50 μL of DEPC water; extraction procedure was performed according to manufacturer’s instruction and cDNA was synthesized as mentioned before.
3.6. RT-PCR Amplification for Enterovirus Detection and Sequencing
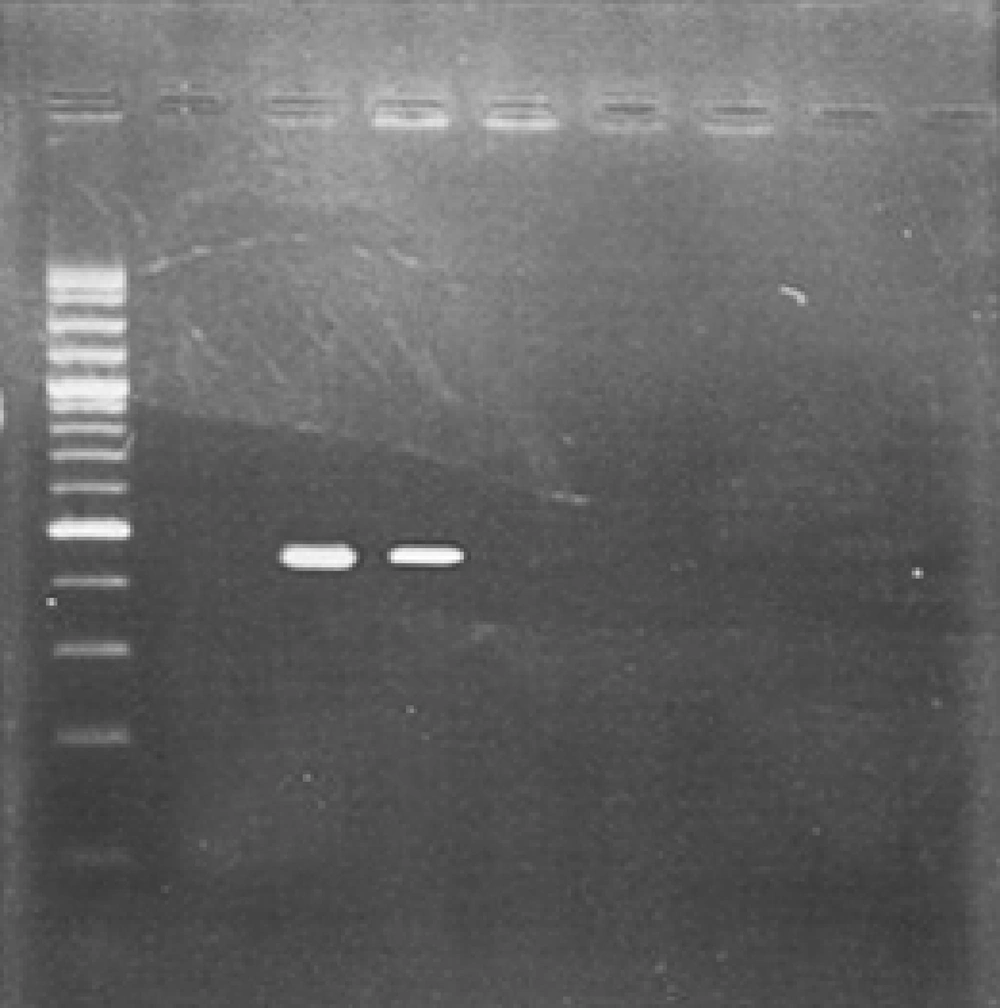
cDNA was synthesized from Cell culture supernatant extract and investigated by RT-PCR with pan enterovirus primers. The specific primers were : EV-1: CAAGCACTTCTGTTTCCCCGG, (5' UTR, 168-188) and EV-R: ATTGTCACCATAAGCAGCCA, (5' UTR, 588-606); these primers amplify an amplicon with 440 bp in length. Amplification were prepared finally in a mixture containing 5µL of cDNA, 5µl 10x viBuffer, 1µL dNTP (10 mM), 0.25µL (1.25 unit) taq polymerase, 3 mM MgCl2, 1.5µL from each primer (0.3 µM), and water up to 50µL (10). The cycling program included incubation at 94ºC for 5 minutes and 35 cycles with denaturation at 94ºC for 1 min, annealing at 53ºC for 1 min, extension at 72ºC for 1 min, and final extension at 72ºC for 5 minutes . 5µL of PCR product was mixed with 6x loading dye and loaded onto 2% w/v agarose gel. After the electrophoresis (100 volts for 50 minutes, 0.5x TBE buffer), the gel was stained in ethidium bromide (0.5 µg/ mL) and amplicon was visualized using UV trans-illuminator (Lambert, France) to observe 440 bp band. PCR product was sent to Bioneer Company (Korea) for sequencing.
3.7. Phylogenic Analysis
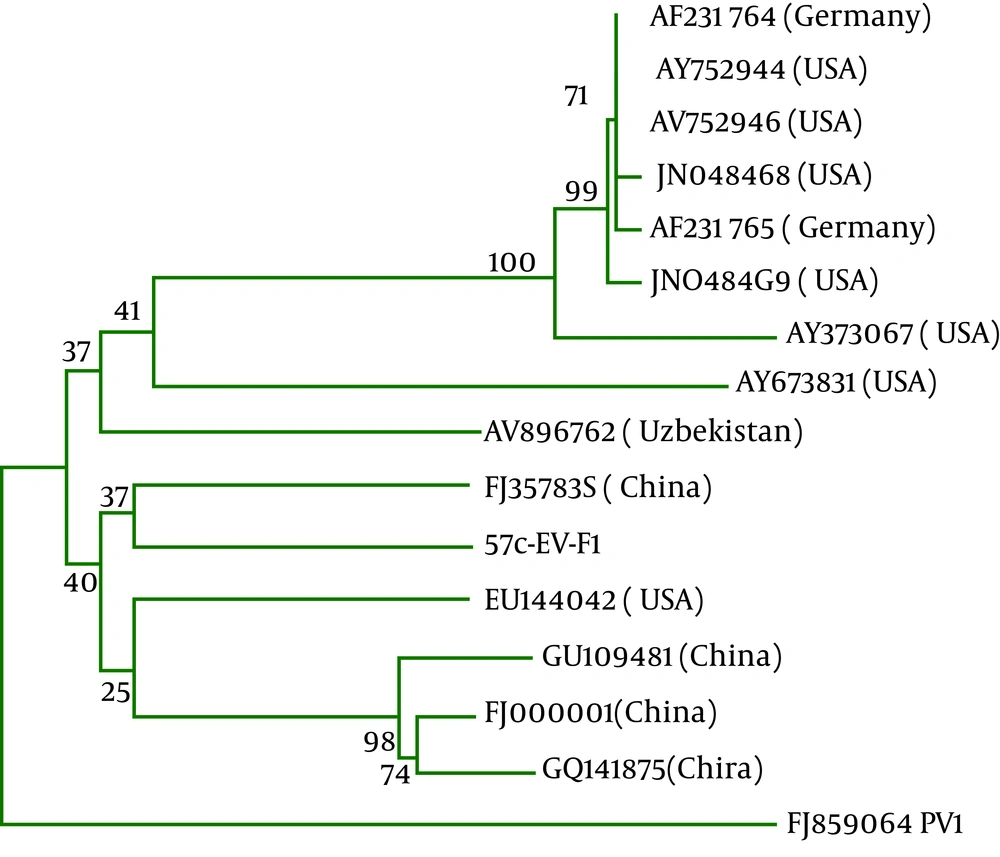
440 bp sequencing result of a positive sample was entered in gene bank and compared with sequences of enterovirus strains using the Basic Local Alignment Search Tool (BLAST). Coxsackie virus B3, Jiangsu strain exhibited a high identity and query coverage with highest total score with our sequence. We prepared a database with our sequences and other coxsackie virus B3 and draw a phylogenic tree using MEGA software version 5 by Neighbor Joining method with 1000 replication bootstrap (Figure 1).
4. Results
In this study, we investigated 34 CSF (cerebrospinal fluid) samples from patients under 14 years old with enterovirus aseptic meningitis using RT-PCR, cell culture, and sequencing tests. More frequent observable symptoms of aseptic meningitis in these patients included fever (78%), seizures (44%), vomiting (44%), and other symptoms that are shown in Table 1 . Patients’ CSF characteristics were also determined; in all of them lymphocytes were dominant; details are shown in Table 2 . After performing RT-PCR to detect echovirus 30 among 34 CSF samples, we didn't find any positive sample.
Thirty four enterovirus positive samples were cultured in RD cell among them only one sample showed the growth that was approved by RT-PCR with pan enterovirus primers of 5'UTR region with 440bp amplicon ( Figure 2 ). Positive sample in culture was sent to Bioneer Company for bidirectional sequencing; it was recognized as coxsackie virus B3. Phylogenic tree analysis of coxsackie virus B3 was drawn by Neighbor Joining method ( Figure 1 ). Based on the tree, this virus is similar to a strain of coxsackie B3 virus from Jiangsu China.
| Symptoms | Total, % | Enterovirus, % |
|---|---|---|
| Fever | 82 | 78 |
| Seizures | 46 | 44 |
| Vomiting | 46 | 44 |
| Fatigue | 28 | 16 |
| Diarrhea | 28 | 22 |
| Neck stiffness | 14 | 11 |
| Hallucination | 18 | 16 |
| Flu like symptom | 11 | - |
| Anorexia | 18 | 22 |
| Headache | 11 | 11 |
| CSF Characteristics | < 1 year | 1 to 2 years | > 2 years | Average |
|---|---|---|---|---|
| WBC Count (/mm3) | 36 (1-172) | 183 (5-540) | 71 (7-125) | 76 (1-540) |
| Lymphocytes | 27 (1-137) | 150 (4-459) | 43 (2-85) | 59 (1-459) |
| Neutrophils | 8 (0-35) | 33 (0-81) | 28 (5-57) | 17 (0-81) |
| Protein, mg/dL | 40 (12-278) | 42 (17-105) | 18 (12-24) | 37 (12-278) |
| Glucose, mg/dL | 97 (15-455) | 70 (25-135) | 79 (58-95) | 88 (15-455) |
5. Discussion
Aseptic meningitis is one of the most prevalent infectious diseases in children and adults that is recognized by clinical symptoms such as fever, headache, neck stiffness, and photophobia (1, 3). Viruses are among major pathogens of aseptic meningitis and the most important genus is enterovirus. Gold standard for diagnosis of enterovirus infection is virus isolation in cell culture, but nowadays most researchers use molecular methods such as RT-PCR that is more sensitive than virus isolation (8).
We didn't detect echovirus 30 in our patients because of some essential features of enteroviruses among them it is notable that coxsackie B5 virus, echovirus type 4, 6, 9, and 30 are famous to create outbreaks and are associated with greater frequency of meningitis than others (7), in previous studies was done in Ahvaz , we didn't see the characteristics of an outbreak (9, 10). High prevalence of enteroviruses in Ahvaz (9, 10) is due to poor hygiene and contaminated water and therefore, we can call it as an endemic area. In such endemic area, echovirus 30 has a low chance to be a major cause of meningitis. Second reason might be attributed to different epidemiology of enteroviruses in different geographical regions and high diversity exists in human enteroviruses.
Our results are similar to two reports from Kuwait and Iran (2, 11). Dalwai's study from Kuwait surveyed enterovirus epidemiology in a three years period, and observed that echovirus 30 was prevalent only in the third year (2). Also, in a study performed in Tehran, Iran by Mirpour et al, echovirus 30 was not observed in patients with aseptic meningitis; all of them were found to have coxsackie B4 and B5 (11). Our results are fully similar to their survey. Decrease in positive cases in cell culture (only one sample) may have several causes. The first one returns to the culture method.
As all enteroviruses cannot be cultured using only one cell type, the method which was performed by us, therefore, we reached to a low positive result. The second reason might be attributed to the existence of antibody in CSF samples that may neutralize virus particles and prevent them to attach to the cell receptors. In different studies, researchers use different kinds of primers to amplify different regions of enterovirus genome. In some studies, VP1 region is used to sequence enteroviruses but in this region, almost all primers are degenerated because of much variety that exists in VP1 region (2); so, in other studies VP2-VP4 regions and 5'UTR region are used for sequencing (2, 12) in which common primers can be used better than that in VP1 region to setup a PCR reaction . The result of our sequencing was coxsackie B3 virus and based on phylogenic tree, it was similar to a strain in Jiangsu, China (10). This result is remarkable because coxsackie B3 virus is a major cause of myocarditis among enteroviruses and, as mentioned in patients section, we recognized a 2-day- old patient who suffered from heart attack.
There was no echovirus 30 in Ahvaz because of diverse nature of enteroviruses and several serotypes with various distribution patterns in different geographical regions, and the fact that echovirus 30 is mostly detected in outbreaks, rather than endemism. Coxsackie virus B3 was responsible for aseptic meningitis in a child in this study. Based on another study conducted in Tehran, Iran, it seems that coxsackie B viruses are among current agents causing enterovirus aseptic meningitis in Iran. Of course we need to perform more studies in Ahvaz and other parts of the country to approve this hypothesis.