1. Background
Bacterium-bacterium interactions, diverse in form and content are widely seen in water column with different microparticles. Over one half of marine bacteria examined so far have displayed antagonistic activity towards other pelagic bacteria. Antimicrobial interactions influence, first, the structure of the microbial community and second, the functioning of microbial cenoses (1). Microbial antagonism results from the effects of antibiotic substances inhibiting the growth of microorganisms or killing them. Bacterial production of secondary metabolites, in particular growth inhibitors, is one of the adaptation mechanisms, which gives advantage in competition for available nutrients and living space.
Searching for previously unknown microbial strains is an effective approach to obtain new biologically active substances. Marine bacteria are producers of unique substances which have never been found in terrestrial organisms (2, 3). Secondary metabolites of microbial origin are widely used in various fields of human activities, such as medicine, agriculture, pharmaceuticals, food processing, chemical industries and many others. In addition to production of antibiotics and lytic enzymes (4), the antimicrobial properties of microorganisms are used to work out biofilms with anticorrosion and antifouling properties (5, 6) and to enhance crop protection against phytopathogenic bacteria (7) and fungi (8).
In aquaculture, the usage of live cultures of antagonistic bacteria as probiotics is developing to prevent outbreaks of diseases in aquatic organisms (9, 10). The interest to biological control in the past two decades has increased dramatically due to expanding opportunities for synthesis of biological products and highly competitive chemical preparations which could often inflict enormous damage to the environment (11).
2. Objectives
The aim of our study was to investigate the peculiarities of bacterium-bacterium antagonistic interactions among heterotrophic bacteria of marine origin in tropical and temperate zones. This would be helpful to clarify some problems concerning the role of biosynthesis of antimicrobial substances in natural bacterial communities and could also be helpful to develop a strategy of search for new physiologically active substances of bacterial origin.
3. Materials and Methods
3.1. Sampling
Heterotrophic bacteria were isolated from different marine objects in Nha Trang Bay, South China Sea in June–July 2008 and January 2009; in the pelagic part of the East China Sea in April 2010; and in Peter the Great Bay, Sea of Japan, in August 2008, 2009 and 2010 in Nha Trang Bay. Invertebrates and algae were collected by scuba divers from depths of three to 23 meters. Microflora samples of the fouling of copper-containing and aluminum plates were selected on a test bench of Marine Corrosion Station, Primorsky Branch of Russian-Vietnamese Tropical Center, Nha Trang (Vietnam). Water samples were taken directly at exposure points of metal plates. The samples of algae included the green alga Caulerpa lentillifera and brown algae Padina spp., Turbullaria spp. and Sargassum spp. The samples of animals included the ascidian Didemnum molle, bivalves Pinctada margaritifera and Crassostrea gigas and unidentified species of sponges (three samples).
3.1.1. Open Part of the East China Sea
Water samples were collected using a Niskin bottle at the points with coordinates 29 º 40, 647 'N, 124 º 27, 909 'E and 26 º 46, 326 'N, 121º 41, 916 'E.
3.1.2. Peter the Great Bay
The samples of algae, animals and water were collected by scuba divers in Avangard Bight of Peter the Great Bay. The examined animals included bivalves Crenomytilus grayanus, Modiolus dificillus and C. gigas; echinoderms, sea urchins Strongylocentrotus nudus and S. intermedius; starfishes Patiria pectinifera and Asterias amurensis, and sea cucumber Apostichopus japonicus and the ascidian Halocynthia aurantium. The seaweed Laminaria japonica was examined from the plants. As test cultures the following type strains were applied: Escherichia coli ATCC 15034, Bacillus subtilis BKM B501, Candida albicans KMM 455, Pseudomonas aeruginosa KMM 433 and Staphylococcus aureus ATCC 21027.
3.2. Isolation of Bacteria in Pure Cultures and Phenotypic Characterization of the Isolates
Isolation of bacteria from hydrobionts and seawater and preservation of bacterial strains were conducted as described elsewhere (12). Bacteria were isolated from internal tissues of ascidians and sponges, coelomic fluid and digestive tract of sea urchins, bivalves and starfishes and digestive tract of holothurians. Biofilm samples (8 cm2 in area) were scrapped off from the surface of each metal plate using a stencil and a sterile tool and then carefully taken with a sterile absorbent cotton stick. The stick with the microbial mass was placed into a test tube containing 2 ml of sterile seawater.
Serial dilutions of homogenates, biofilm suspensions and water samples (0.1 ml) were plated on solid Youschimizu–Kimura medium (13) with the following composition: peptone (5.0 g), yeast extract (2.0 g), glucose (1.0 g), К2НРО4 (0.2 g), MgSO4•7H2O (0.1 g), agar (12.0 g), distilled water (500 ml), and seawater (500 ml); the рН of the medium equaled 7.8 to 8.0. Cetrimide agar (Serva) supplemented with glycerol in proportion of 10 g/l was used to isolate bacteria of the genus Pseudomonas. The plates were incubated for three to ten days at 28 °С for tropical isolates and at 23 °С for isolates from temperate zone.E. coli, C. albicans, S. aureus, P. aeruginosa and B. subtilis were cultured on tryptic soya agar (TSA, Difco).
Motility and cell morphology were observed by the hanging-drop method. Gram-reaction, oxidase and catalase activities, presence of nitrate reductase, sodium ion requirements and tolerance to different NaCl concentrations (0–12% NaCl), growth at different temperatures (4–42 °C), acid production from sugars, production of lysine and ornithine decarboxylases and arginine dehydrolase and gelatinase, DNA base composition and resistance to antibiotics were tested as described elsewhere (14). The following antibiotics were used for tests: ampicillin (Amp), erythromycin (Ery), gentamicin (Gen), lincomycin (Lin), rifampicin (Rif), oleandomycin (Ol), polymyxin B (Pol) and vibriostatic agent O-129 (2,4-diamino-6,7-di-isopropylpteridine) with Oxoid disks. Additional biochemical tests with API-20NE and API-20E test kits (bioMérieux) were performed as described by the manufacturers, except that strains were suspended in 3% NaCl.
3.3. 16S rRNA Gene Sequence Analysis of Bacterial Isolates
Total DNA was isolated using the standard technique (15). A fragment of 16S rRNA gene sequence was amplified in 25 µl of reaction mixture comprising 2.5 µl of 10 × PCR buffer, 2 µl of 10 mM dNTP mixture (2.5 mM each), 2.5 µl of each primer (2.5 μM), 10 ng of DNA and 1 unit of Taq DNA polymerase (Fermentas). Primers amplification were performed according to Lane (16). The PCR amplification (GeneAmp PCR System 9700, Applied Biosystems) was performed using the following scheme: an initialization hold at 95 °C for three minutes, 35 cycles each comprising 30 seconds at 94 °C, one minute at 56 °C and 1.5 minutes at 72 °C and the final hold at 72 °C for five minutes.
The purity and size of products were estimated in 1% agarose gel. The purified amplification products were applied as a matrix for sequencing, which was performed with a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). The purified products of sequencing were subjected to electrophoresis with an ABI Prism 3130 genetic analyzer, on a 50 cm capillary cartridge. The obtained direct and inverse sequences for each tested species were aligned using SeqScape v2.6 (Applied Biosystems) software. The obtained fragments of 16S rRNA gene sequences were deposited in NCBI/GenBank (GenBank accession numbers. GU579451, GU579452, GU726840–GU726880, JN679843–JN679865). Phylogenetic trees were developed with the Neighbor-Joining Method (NJ) (17), using Kimura's two-parametric model of nucleotide substitutions (K2P) (18) and MEGA 5 software (19). Cluster stability was estimated using bootstrap analysis (1000 iterations) (20).
3.4. Screening of Isolates for Inhibitory Interactions
Antimicrobial activity was tested in strains isolated in Vietnam from the surface of metal plates fouling and seawater and in Russian isolates associated either with different hydrobionts or with free-living in seawater. As test cultures strains were used isolates in tropical and temperate zones from seawater and different hydrobionts and five type strains belonging to Gram-positive bacteria, Gram-negative bacteria and yeasts. Antimicrobial activity of the isolates was assayed with a slightly modified method of Long and Azam (1). A lawn of a target isolate was prepared by mixing 25 ml of molten (44 °C), 0.6% Marine agar with 0.5 ml of isolate suspension. The suspension was prepared by dilution of an MA-grown daily culture in physiological solution down to concentration of 109 cells/ml, according to the McFarland Turbidity Standard. From 12 to 16 strains of potential growth inhibitors were spotted on the lawn. The plates were incubated face up for six days at 28 °С for tropical isolates and at 23 °С for temperate zone and examined daily for zones of inhibition. Potential producers were considered positive, if the diameter of the inhibition zone was at least 4 mm greater than that of the colony formed by the potential producer.
4. Results
4.1. Identification of Bacteria
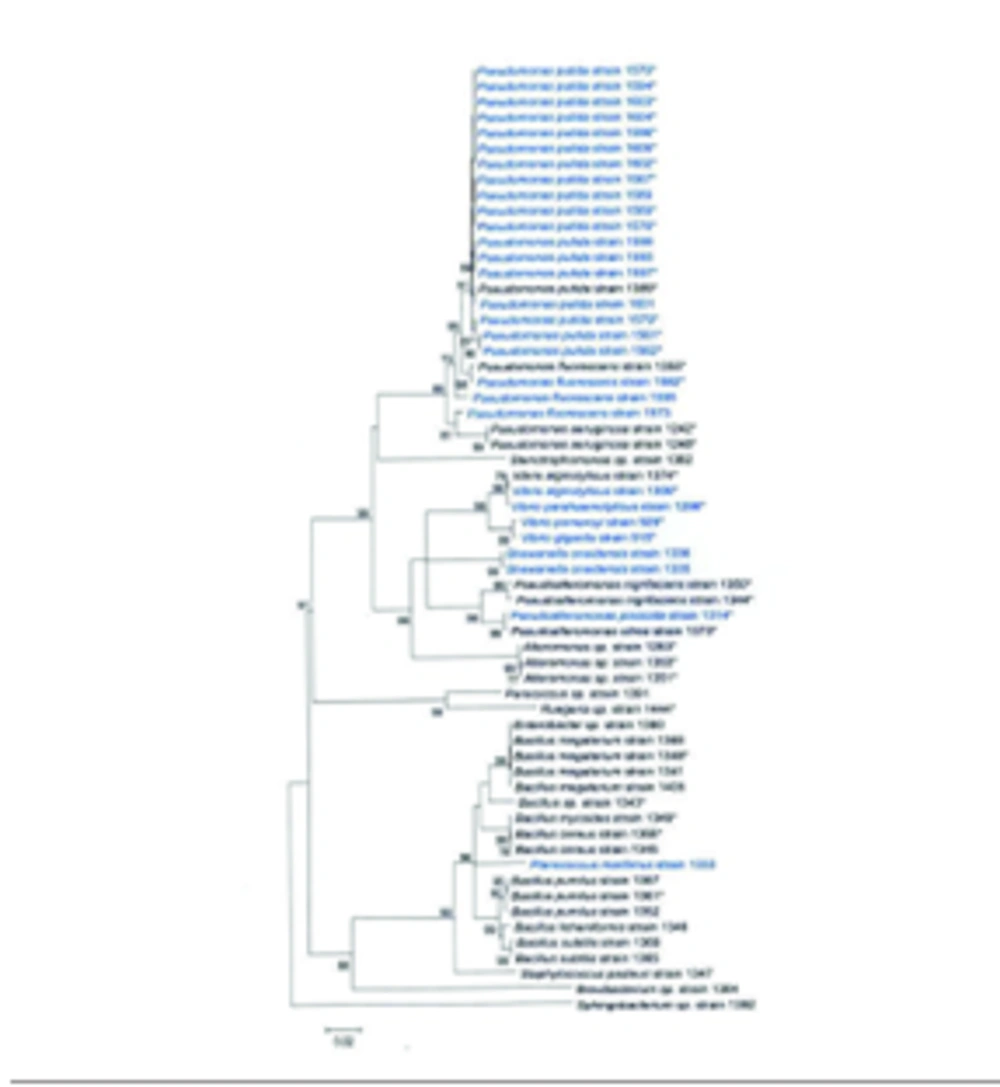
Among 66 strains analyzed for antimicrobial activity, 61 strains were identified using results of 16S rRNA gene sequencing ( Figure ).
The strains used as test cultures and five antagonistic strains were identified by phenotypic characteristics. The obtained data was compared with available literature to corroborate the identification of some bacterial strains. To specify the taxonomic position of some strains, antibiotics sensitivity test was applied. For example, the strain 1541 Pseudoalteromonas sp, tested for antimicrobial activity, showed oxidative metabolism pattern, possessed cytochrome oxidase, demonstrated low G/C DNA molar percentage ratio (39.5%) and had dark brown pigment which diffused into the medium; it hydrolyzed agar and did not possess nitrate reductase. It was sensitive to rifampicin, ampicillin, erythromycin, gentamicin and polymyxin, but resistant to lincomycin and oleandomycin.
Proceeding from phylogenetic and phenotypic analysis the strains examined for antimicrobial activity were divided into five different phylogenetic groups as follow: γ- Proteobacteria (66.7%), Firmicutes (27.3%), α-Proteobacteria (3%), Actinobacteria (1.5%), and Bacteroidetes (1.5%). Most of γ-Proteobacteria belonged to genera Pseudomonas and Pseudoalteromonas; bacteria of genera Vibrio, Shewanella, Alteromonas, Enterobacter, and Stenotrophomonas were registered as minor groups ( Table 1 ). Firmicutes mostly constituted of species of the genus Bacillus and also included significantly fewer members of genera Staphylococcus and Planococcus.
The group of α-Proteobacteria included genera Ruegeria and Paracoccus; while phyla Bacteroidetes and Actinobacteria included one registered member each, namely the genera Sphingobacterium and Brevibacterium, respectively. Most Russian isolates obtained from different hydrobionts were categorized as γ- Proteobacteria, whereas more than a half of tropical isolates from the surface of copper-containing plates belonged to Firmicutes. Most of 21 strains identified by phenotypic characteristics belonged to genera Bacillus and Vibrio ( Table 2 ). Genera Xanthomonas, Enterobacter, Planococcus and Serratia included one registered species each. The taxonomic position of strains 1335 and 1336 (S. oneidensis) and the strain 1355 (P. fluorescens), which were examined for capability to produce antimicrobial compounds and also were used as test cultures was determined using the results of 16S rRNA gene sequencing.
| Strain No./ Gene Bank Ref. No. | Source of Isolation | Close Phylogenetic Relatives | Identity, % |
|---|---|---|---|
| Vietnam, Nha Trang Bay, South China Sea | |||
| 1242/GU726840 a | brass | P | 100 |
| 1248/GU726841 | brass | P. aeruginosa | 100 |
| 1263/GU726843 | bronze | Alteromonas sp. | 100 |
| 1341/GU726850 | brass | B | 100 |
| 1343/GU726852 | brass | Bacillus sp. | 99,6 |
| 1344/GU726853 | brass | P | 96,6 |
| 1345/GU726854 | brass | B | 100 |
| 1346/GU726855 | brass | B | 100 |
| 1347/GU726856 | brass | S | 100 |
| 1348/GU726857 | brass | B | 100 |
| 1349/GU726858 | brass | B | 99,8 |
| 1350/GU726859 | bronze | P | 97,3 |
| 1351/GU726860 | bronze | Alteromonas sp. | 99.9 |
| 1352/GU726861 | bronze | B | 100 |
| 1353/GU726862 | bronze | Alteromonas sp. | 99,9 |
| 1355/GU726863 | copper | P | 100 |
| 1360/GU726864 | copper | Enterobacter sp. | 99,5 |
| 1361/GU726865 | copper | B | 100 |
| 1364/GU726866 | copper | Brevibacterium sp. | 99,9 |
| 1365/GU726867 | copper | B | 100 |
| 1366/GU726868 | copper | B | 99,9 |
| 1367/GU726869 | brass | B | 100 |
| 1368/GU726870 | brass | B | 100 |
| 1369/GU726871 | brass | B | 100 |
| 1373/GU726872 | brass | P | 99,9 |
| 1374/GU726873 | seawater | V | 99,6 |
| 1382/GU726874 | aluminum | Stenotrophomonas sp | 100 |
| 1389/ | aluminum | P | 100 |
| 1391/GU726876 | copper | Paracoccus sp. | 99,1 |
| 1392/GU726877 | brass | Sphingobacterium sp. | 99,3 |
| 1405/GU726879 | sponge | B | 99,9 |
| 1441 | seawater | Staphylococcus sp. | n/d b |
| 1442 | seawater | Staphylococcus sp. | n/d |
| 1443 | seawater | Vibrio sp. | n/d |
| Vietnam, Nha Trang Bay, South China Sea; The East China Sea | |||
| 1444 | seawater | Ruegeria sp. | 99,9 |
| 1541 | seawater | P | n/d |
| 1542 | seawater | Vibrio sp. | n/d |
| Peter the Great Bay, Japan Sea, Russia | |||
| 915/EU579451 | trepang | V | 99.8 |
| 929 | trepang | V | 99.1 |
| 1298/GU726844 | sea urchin | V | 99,9 |
| 1306/GU726845 | laminaria | V | 99,9 |
| 1314/GU726846 | soil | P | 100 |
| 1333 | ouster | P | 99,6 |
| 1335/GU726848 | ouster | S | 99,8 |
| 1336/GU726849 | ouster | S | 99,8 |
| 1561 | seawater | P | 99,5 |
| 1562 | seawater | P | 99,5 |
| 1567 | starfish | P | 99.9 |
| 1569/JN679848 | starfish | P | 99.9 |
| 1570 | sea urchin | P | 99.9 |
| 1573 | starfish | P | 97.3 |
| 1576 | starfish | P | 100 |
| 1579 | bivalve | P | 99.2 |
| 1582 | starfish | P | 99.6 |
| 1589 | starfish | P | 99.9 |
| 1593 | starfish | P | 99.9 |
| 1594 | sea urchin | P | 99.9 |
| 1595 | trepang | P | 97.1 |
| 1596 | bivalve | P | 99.9 |
| 1597 | holothurian | P | 99.9 |
| 1599 | bivalve | P | 99,9 |
| 1601 | bivalve | P | 99.8 |
| 1602 | sea urchin | P | 99.9 |
| 1603 | sea urchin | P | 99.9 |
| 1604 | sea urchin | P | 99.9 |
| 1605 | sea urchin | P | 99.9 |
aThe numbers of 16S rRNA gene sequences deposited in NCBI/GenBank
bno data
| Strain No. | Source of Strain | Close Phylogenetic Relative | Identity, % |
|---|---|---|---|
| 1420 | Vietnam, holothurian | Bacillus | n/d |
| 1421 | Vietnam, holothurian | Bacillus | n/d |
| 1422 | Vietnam, holothurian | Bacillus | n/d |
| 1423 | Vietnam, holothurian | Vibrio | n/d |
| 1424 | Vietnam, holothurian | Vibrio | n/d |
| 1425 | Vietnam, holothurian | Vibrio | n/d |
| 1427 | Vietnam, ouster | Vibrio | n/d |
| 1430 | Vietnam, algae | Bacillus | n/d |
| 1437 | Vietnam, algae | Bacillus | n/d |
| 1438 | Vietnam, algae | Bacillus | n/d |
| 1478 | Vietnam, seawater | Bacillus | n/d |
| 1537 | Vietnam, seawater | Vibrio | n/d |
| 1543 | Vietnam, seawater | Xanthomonas | n/d |
| 1306 / GU726845 | Russia, laminaria | V | 99.9 |
| 1331 | Russia, trepang | Enterobacter sp. | n/d |
| 1333 / GU726847 | Russia, ouster | P | 99.6 |
| 1335 | Russia, ouster | S | 99.8 |
| 1336 | Russia, ouster | S | 99.8 |
| 1355 | Vietnam, copper | P | 100 |
| 1410 | Vietnam, aluminum | S | 99.9 |
| 1530 | Vietnam, bivalve | Vibrio sp. | n/d |
| ATCC 15034 | КММ, PIBOC* | E | n/d |
| КММ 455 | КММ, PIBOC | C | n/d |
| ATCC 21027 | КММ, PIBOC | S | n/d |
| B | КММ, PIBOC | B | n/d |
| КММ 4 | КММ, PIBOC | P | n/d |
The strains are deposited in the Collection of Marine Microorganisms (KMM) of the Pacific Institute of Bioorganic Chemistry, Far Eastern Division, Russian Academy of Sciences, Vladivostok, Russia
4.2. Antimicrobial Activity
68.97% of isolates from temperate zone and 56.76% of Vietnamese strains showed antimicrobial activity. The strains that showed the greatest activity were of tropical origin ( Table 3 ). Regarding taxonomic position of the most active strains, in both temperate and tropical zones, the undisputed leaders belonged to families Pseudomonadaceae and Pseudoalteromonadaceae (Table 3 and Table 4). Among Vietnamese isolates the activity against most test cultures, besides P. aeruginosa and P. nigrifaciens, was also demonstrated by Ruegeria sp. and Bacillus spp.
All tropical strains of Alteromonas spp., Pseudomonas spp., Pseudoalteromonas spp. and Vibrio spp. tested for antimicrobial activity appeared active against two or more test cultures. 35.7% of the genus Bacillus members showed antimicrobial activity . Two strains of Russian origin, P. putida no. 1567 and no. 1602, suppressed growth in 10 and 11 test cultures respectively (Table 4). Among pseudomonads 71.4% of strains demonstrated antimicrobial activity. A strain of P. piscicida suppressed growth in 10 test cultures. All Vibrio strains showed antimicrobial activity in respect to 1–4 test cultures. No antimicrobial activity was detected in the examined strains of S. oneidensis and P. maritimus and certain strains of Pseudomonas spp.
Vietnamese isolates were the most active against bacteria of genera Bacillus and Vibrio; Russian strains showed the greatest activity against Bacillus spp., S. marcescens, S. oneidensis and Vibrio spp. Tropical isolates suppressed more actively the growth of E. coli and S. aureus, whereas strains from temperate area were more active against P. aeruginosa. None of Russian isolates showed activity against P. fluorescens and C. albicans, whereas tropical strains of P. citrea and Ruegeria sp. suppressed the growth of P. fluorescens, while P. nigrifaciens and Alteromonas sp. were active against C. albicans.
| 1420 | 1421 | 1422 | 1423 | 1424 | 1425 | 1427 | 1430 | 1437 | 1438 | 1478 | 1537 | 1543 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1242 | + a | + | + | + | - | + | + | + | + | - | + | + | + |
| 1248 | + | + | + | + | + | + | + | + | - | - | + | + | + |
| 1263 | - b | - | - | - | - | - | + | - | - | - | - | - | - |
| 1343 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1344 | + | + | + | + | - | - | - | + | - | - | + | - | - |
| 1348 | + | - | - | - | - | - | + | - | - | - | - | - | - |
| 1349 | - | + | - | - | - | - | + | - | - | - | - | - | - |
| 1350 | + | - | - | + | - | + | - | + | + | - | + | - | - |
| 1351 | - | - | - | - | - | - | + | - | - | - | - | + | - |
| 1353 | + | - | - | + | - | - | - | + | - | - | - | - | - |
| 1355 | - | - | - | - | - | - | - | + | + | - | - | - | - |
| 1361 | + | - | + | - | - | + | - | - | - | - | - | - | + |
| 1366 | - | - | - | - | - | - | + | - | - | - | + | + | - |
| 1373 | - | - | - | - | + | - | - | + | - | - | - | - | + |
| 1374 | - | - | - | - | - | - | + | - | - | - | + | - | - |
| 1389 | + | - | - | + | - | - | - | - | - | - | + | - | - |
| 1442 | - | - | - | - | - | - | - | - | - | + | - | - | - |
| 1443 | + | + | - | - | + | - | - | - | - | - | - | - | - |
| 1444 | + | + | + | + | - | - | + | - | + | + | + | - | - |
| 1541 | - | + | - | + | - | - | - | + | + | - | - | - | - |
| 1542 | - | - | - | - | - | - | - | + | - | + | - | - | - |
| E. coli | C. albicans | S. aureus | P. aeruginosa | B.subtilis | 1306 | 1331 | 1333 | 1335 | 1336 | 1355 | 1410 | 1530 | |
| 1242 | - | - | + | - | + | + | + | - | + | + | - | + | + |
| 1248 | - | - | + | - | + | + | + | - | + | - | - | + | + |
| 1263 | - | - | - | - | - | + | - | - | - | - | - | - | + |
| 1343 | + | - | - | - | - | - | + | - | - | - | - | - | - |
| 1344 | - | + | - | + | - | - | - | - | - | - | - | - | - |
| 1348 | + | - | - | - | + | - | - | - | - | - | - | - | - |
| 1349 | - | - | + | - | - | - | + | - | - | - | - | + | - |
| 1350 | - | - | + | - | - | - | - | + | - | - | - | - | - |
| 1351 | - | - | - | - | - | + | - | - | - | - | - | - | - |
| 1353 | - | + | - | - | - | - | - | - | - | - | - | - | - |
| 1355 | + | - | - | - | + | - | + | - | - | - | n/d c | + | + |
| 1361 | + | - | + | - | - | - | - | - | - | - | - | - | - |
| 1366 | + | - | - | - | - | + | - | - | + | - | - | - | + |
| 1373 | - | - | - | - | - | - | - | - | - | + | + | - | + |
| 1374 | - | - | - | - | - | - | - | - | - | - | - | - | + |
| 1389 | + | - | - | - | - | - | - | - | - | - | - | + | - |
| 1442 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1443 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1444 | + | - | + | - | - | - | - | + | + | + | + | - | - |
| 1541 | - | - | - | - | - | - | - | - | + | + | - | - | - |
| 1542 | - | - | - | - | - | - | - | - | - | - | - | - | - |
aGrown inhibition zone was more than 5 mm in diameter
bGrown inhibition zone was absent
cn/d – no data
| 1420 | 1421 | 1422 | 1423 | 1424 | 1425 | 1427 | 1430 | 1437 | 1438 | 1478 | 1537 | 1543 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 915 | - | - | - | - | - | - | + | - | - | - | - | - | - |
| 929 | - | - | - | + | - | + | - | - | - | - | - | + | - |
| 1298 | - | - | - | - | - | - | + | - | - | - | - | + | - |
| 1306 | - | - | - | - | - | - | - | - | - | - | - | + | - |
| 1314 | - | - | - | + | - | - | + | - | - | - | + | + | - |
| 1561 | - | - | - | - | - | + | - | - | - | - | - | + | - |
| 1562 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1567 | + | + | + | + | - | - | - | + | - | + | + | - | - |
| 1569 | - | + | + | - | - | + | - | + | + | + | - | - | + |
| 1570 | - | - | - | - | - | - | - | + | + | - | - | + | - |
| 1576 | - | + | - | - | - | - | - | - | - | - | - | - | + |
| 1579 | - | + | - | - | - | - | - | - | - | - | + | - | - |
| 1582 | + | + | - | - | - | - | - | - | - | - | - | - | - |
| 1594 | + | + | + | - | - | - | - | + | + | + | - | - | - |
| 1596 | - | + | - | - | + | - | - | + | - | - | - | - | - |
| 1597 | - | - | - | - | - | - | - | - | - | - | - | - | + |
| 1602 | + | + | + | - | + | - | - | + | + | + | - | - | - |
| 1603 | - | + | + | - | + | - | - | + | - | - | - | - | - |
| 1604 | - | + | + | - | + | - | - | + | - | + | - | - | - |
| 1605 | + | - | + | - | + | - | - | + | - | - | - | - | - |
| 915 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 929 | - | - | - | - | - | + | - | - | - | - | - | - | - |
| 1298 | - | - | - | - | - | + | - | - | - | - | - | - | + |
| 1306 | - | - | - | - | - | n/d | - | - | - | - | - | - | - |
| 1314 | + | - | + | - | - | - | + | - | + | - | - | + | + |
| 1561 | - | - | - | - | + | + | + | - | + | - | - | + | + |
| 1562 | - | - | + | - | + | + | + | + | - | - | - | + | - |
| 1567 | - | - | - | - | + | + | - | + | - | - | - | - | - |
| 1569 | - | - | - | - | - | + | + | + | - | - | - | - | - |
| 1570 | - | - | - | - | - | + | - | - | + | + | - | + | + |
| 1576 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1579 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1582 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1594 | - | - | - | - | - | - | - | - | + | - | - | - | - |
| 1596 | - | - | - | - | - | - | - | - | + | - | - | + | - |
| 1597 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1602 | - | - | - | + | - | - | - | - | + | + | - | + | - |
| 1603 | - | - | - | + | - | - | - | - | + | + | - | - | - |
| 1604 | - | - | - | + | - | - | - | - | + | - | - | - | - |
| 1605 | - | - | - | + | - | - | - | - | - | + | - | - | - |