1. Background
In the past two decades, the prevalence of candidiasis has been increased. Candida species are able to create superficial and systemic infections. Candida albicans is an opportunistic pathogen, causing mycoses in immunocompromised patients as well as long-term antibiotic users (1). Also, other Candida species such as C. glabrata, C. parapsilosis, C. tropicalis and C. krusei are among the oral mucosal lesions suspected agents in AIDS patients (2). Herbal Medicines have been used as alternative drugs in developing countries. Brazil, Cuba, India, Jordan and Mexico are examples of countries with various herbs as well as a potent folklore in using medicinal plants for their antimicrobial and antifungal benefits (3-6). Heracleum persicum (in the Apiaceae family), known as Golpar in Persian, is vernacular to Iran. It grows wildly in humid mountainous regions and is used in soups and stews. Ten out of 70 species (7) of H. persicum are known in Iran (8). H. persicum fruit is extensively used as spice and the young stems are also used for making pickles. Chemical compounds such as Pimpinellin, isopimpinellin, bergapten, isobergapten and six furanocoumarins have been reported to be extracted from its roots (9, 10).
2. Objectives
Because of the high usage of H. persicum fruit as an herbal medicine in the Iranian culture beside its analgesic activity, we decided to survey the hydroalcoholic extract of H. persicum fruit, assessing its anti-Candida activity by agar well diffusion method.
3. Materials and Methods
3.1. Plant Ingridients and Preparation of Hydroalcoholic Extract
H. persicum fruits were purchased from a local market in Ahvaz, Iran. For preparation of hydroalcoholic extract, 10 g air-dried and powdered fruit of H. persicum was macerated with 100 mL of 80% ethanol and methanol on a rotary shaker for 72 hours, filtered, and then the solution was evaporated in the room temperature. Dried hydroalcoholic extract was stored in a sterile glass bottle at -20°C until future assays. One gram of the dried hydroalcoholic extract of H. persicum was dissolved in 5 mL dimthyl sulphoxide (DMSO, 100%) to a final concentration of 200 µg/µL as stock, and serial double fold dilutions were prepared using sterile distilled water from 0.078 - 40 µg/µL according to the previous literature (11).
3.2. Yeast Inoculum Preparation
A total of 47 Candida spp. isolates including C. albicans [29], C. glabrata [10], and C. tropicalis [7], isolated from oral swabs samples, were selected. Candida spp. isolates were inoculated into Sabouraud dextrose broth (SDB, Merk, Germany) and grown overnight on a rotary shaker at room temperature. Then cells were washed three times with sterile distilled water and adjusted by the same solvent to yeast inoculum of 106 CFU/mL (0.5 Mac-Farland standard).
3.3. Positive and Negative Controls
The commercial antifungal drugs such as clotrimazole disc (10 µg/disc) and nystatin disc (100 IU/disc) were used as positive controls and DMSO was used as negative control.
3.4. Anti-Candida Assay
Anti-Candida activities of the hydroalcoholic extracts of H. persicum fruit were assayed against Candida spp. isolates by agar well diffusion according to Perez et al. method (12). One hundred microliter of yeast inoculum (106 cells/mL) was uniformly spread onto Sabouraud dextrose agar medium (SDA, Merck, Germany) using a bent glass rod. Then five wells of 7 mm diameter were punched by a borer into the SDA medium and filled with 100 µL of two-fold serial dilutions of plant extracts as well as sterile DMSO 100% as negative control. Plates were incubated for 24 hours at 37°C. Anti-Candida activity was determined by measuring the zone of inhibition. Experiments were carried out three times. Two positive controls such as clotrimazole and nystatin discs were placed in the plate. The lowest concentration of a tested plant extract exhibiting a clear zone, was considered as the minimum inhibitory concentration (MIC).
4. Results and Discussion
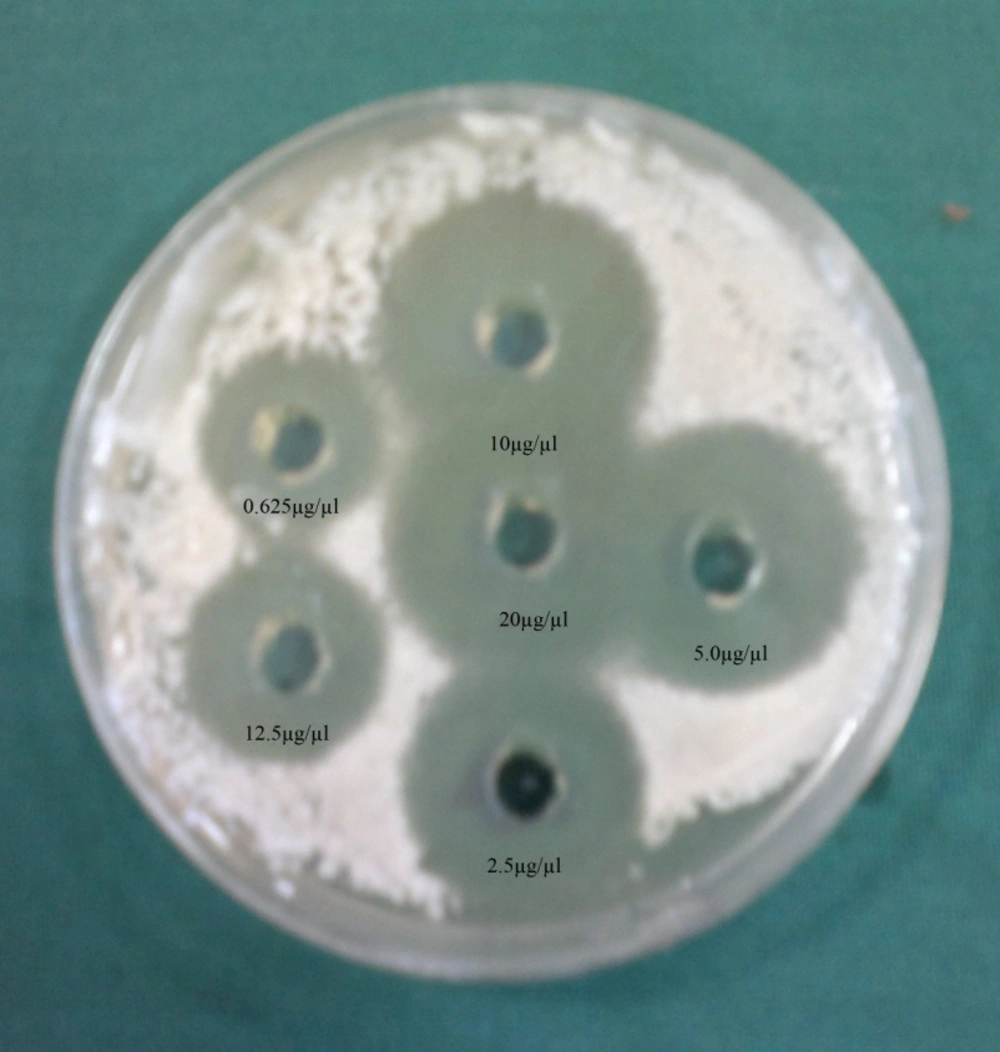
In the current study, we surveyed the anti-Candida activity of the hydroalcoholic extracts of H. persicum fruit against three different Candida species (C. albicans , C. tropicalis and C. glabrata). The results are summarized in Figure 1 and Tables 1 and 2. The ethanolic and methanolic extracts of the tested plants showed anti-Candida activities against C. albicans (n = 29), C. tropicalis (n = 10), and C. glabrata (n = 7). The strongest activity was seen against C. albicans with a range of 12 - 21 mm inhibition zones and 0.625 - 20 µg/µL MIC values; the ethanolic extract of the tested plant has more anti-Candida effects at 0.625 µg/µL compared to the methanolic extract at 2.5 µg/µL. Similar results were reported by other researchers with different medicinal plants (13). According to Mimica-Dukic et al. study (14) among tested Candida spp. isolates, C. albicans was the most sensitive tested Candida spp. to Mentha piperita L. oil, which was in agreement with our results. The lowest concentration of the tested plant showed a potential anti-Candida activity against C. albicans and C. glabrata , while the highest concentration showed a weak inhibitory effect against C. tropicais . Furthermore, previous studies reported that the essential oil of H. persicum has moderate anti-Candida activity (15). In Iranian traditional medicine, H. persicum fruit was used as a carminative and pain relieving herbal drug ( 16 , 17 ). Review of literature reported that coumarins were known to be responsible for antifungal activity of many of the medicinal plants (18, 19). It was suggested that furanocoumarins, isolated from the fruit of H. persicum (10), were the cause of anti-Candida activity of this plant. Candida species are in the normal flora of healthy people, while they can cause superficial mycosis and invasive infections in immunocompromised patients (20). During the past 20 years, the incidence of infection by pathogenic fungi has been increased; most of which were infected by Candida spp. that can change the superficial mycosis to invasive infections. C. albicans has been a major factor of morbidity and mortality in immunocompromised patients, but other Candida spp. such as C. glabrata and C. krusei have expanded in the recent years (21 - 23).
| Antifungal Agents | Candida spp. Isolates MIC a, µg/µL | ||
|---|---|---|---|
| C. albicans, No. 29 | C. glabrata, No. 10 | C. tropicalis, No. 7 | |
| Treatments b | |||
| Ethanolicextract of H. persicum | 0.625 - 20 | 0.625 - 40 | 5 - 20 |
| Methanolic extract of H. persicum | 5 - 20 | 2.5 - 20 | 5 - 20 |
| Positive controls | |||
| Clotrimazole | 0.0078 µg/µL | -- c | -- |
| Nysatatin | 0.0039 µg/µL | -- | -- |
aAbbreviation: MIC, minimal inhibitory concentration.
bTests were done in triplicate. Tested concentrations: extracts, 40 µg/µL; positive controls, Clotrimazole 2 µg per well and Nysatatin 2 µg per well.
cno data is provided.
aTests were done in triplicate. Tested concentrations: extracts, 40 µg/µL; positive controls, Clotrimazole 10 µg/disc and Nysatatin 100 IU/disc.
bno data is provided.
Therefore, there is an urgent necessity for finding alternative antifungal drugs for effective treatment of Candidal infections.