1. Background
Rhodotorula species are classified in to the fungal family Sporidiobolaceae (Phylum Basidiomycota) (1). They have a widespread distribution in the environment and are frequently isolated from soil and its products. Rhodotorula species are common airborne contaminant fungi. In addition these species are also considered as normal inhabitants of the skin, lungs, urine and feces in humans (2). In a study conducted by Ruiz-Aragón, et al. Rhodotorula glutinis was the commonest isolated species both in clinical and environmental samples followed by R. minuta and R. mucilaginosa(R. rubra) (2). The genus Rhodotorula includes 34 species, with R. glutinis being the most prevalent species (3). The most common species of Rhodotorula, include; R. glutinis,R. mucilaginosa and R. minuta (4, 5). In addition some species of Rhodotorula (R. mucilaginosa) are used as biological controls for protecting plants and fruits against Botrytis cinerea (6) and biodegradation organic compounds (7).
This species is considered as a non-pathogenic yeast; during last two decades several species of Rhodotorula have been associated with invasive mycosis among immunocompromised patients (8). The most common infections due to Rhodotorula species in the literature are fungemia associated with catheters (9-12), endocarditis (9), peritonitis (9), meningitis (9, 13), keratomycosis (14), dacryocystitis (15), and endophthalmitis (13). In a systematic review 128 Rhodotorula infections were studied by Tuon and Costa. They found that 79% of cases were fungemia followed by eye infections and peritonitis. R. mucilaginosa infecting 74% of cases was the most common agent of infection, followed by R. glutinis (7.7%) and unidentified (17%) (16).
Both amphotericin B and flucytosine have good activity against Rhodotorulain vitro, whereas fluconazole is inactive (8, 17). Several studies show that empirical treatment of Rhodotorula systemic infection is administration of amphotericin B or azoles compounds with or without flucytosine (8). In addition, new antifungal agents such as voriconazole, ravuconazole and posaconazole are active against Rhodotorula species in vitro and are candidates for the treatment rhodotorulosis (18, 19).
2. Objectives
In spite of the increased number of invasive infections due to Rhodotorula spp. during recent years, there have only been a few available data in the literature on the isolation and antifungal susceptibility of this species. In addition limited data on environmental sources of Rhodotorula species in hospitals are available. Therefore the aim of present study was the isolation and identification of Rhodotorula species from two educational hospitals affiliated to Ahvaz Jundishapur University of Medical Sciences. In addition isolated yeasts were evaluated against several antifungal drugs including; amphotericin B, nystatin, miconazole, clotrimazole, fluconazole and terbinafine.
3. Materials and Methods
3.1. Isolation and Identification of Rhodotorula
In the present study, based on the 14% frequency of isolation of Rhodotorula species, 600 samples were collected ( 20 , 21 ). A total of 600 samples were collected from different wards environments and equipment of two educational hospitals in Ahvaz, such as the operating rooms, wards (normal, protective, and critical and intensive care units), outpatient, patient clothes and beds, patients room furniture, uniforms (nurses, doctors, students and staff in the kitchen), floor, walls, windows, and storage. In addition, devices used by patients, medical equipment, trollies, door handles, water taps, computer keyboards and mouse, refrigerators and personnel’s hands were also sampled (Table 1).
| Sampled Sites | Total Samples, No. (%) | Positive Cases, No. (%) | Frequency, % |
|---|---|---|---|
| Patient hands | 30 (5.0) | 0 (0.0) | 0.0 |
| Serum set and blood bags | 61 (10.2) | 3 (7.7) | 4.9 |
| Patient beds | 57 (9.5) | 0 (0.0) | 0.0 |
| Phones and mobile phones | 4 (0.7) | 1 (2.6) | 25.0 |
| Door handles | 16 (2.7) | 0 (0.0) | 0 |
| Floor, walls and windows | 43 (7.1) | 10 (25.6) | 23.3 |
| Nurses hands | 17 (2.8) | 0 (0.0) | 0.0 |
| Nurses stations | 47 (7.8) | 0 (0.0) | 0.0 |
| Keyboards and mouse | 17 (2.8) | 1 (2.6) | 5.9 |
| Medical instruments | 97 (16.2) | 3 (7.7) | 3.1 |
| Nurses uniforms | 22 (3.7) | 3 (7.7) | 13.6 |
| Water taps | 41 (6.8) | 5 (12.8) | 12.2 |
| Hand wash and toilet paper | 27 (4.5) | 0 (0.0) | 0.0 |
| Patient room furniture | 56 (9.3) | 6 (15.4) | 10.7 |
| Refrigerators | 36 (6.0) | 5 (12.8) | 13.9 |
| Recycle bins | 14 (2.3) | 2 (5.1) | 14.3 |
| Patient uniforms | 5 (0.8) | 0 (0.0) | 0.0 |
| Others | 10 (1.7) | 0 (0.0) | 0.0 |
| Total | 600 (100.0) | 39 (100.0) | 6.5 |
Frequency of Rhodotorula Species Isolated From Different Sites of Two Hospitals in Ahvaz
The sampling was carried out by wet and sterile cotton swabs. The cotton swab was drawn on the studied surfaces and then inoculated on Sabouraud dextrose agar (SDA, Merck, Germany) plates containing chloramphenicol. All culture media were immediately transferred to the Medical Mycology Laboratory and were incubated at room temperature for one week. During incubation times, all red-orange yeast colonies were selected and their morphology was confirmed by a microscopic examination. In the present study we recovered 72 strains of Rhodotorula . Yeasts were identified by a commercial system ID 32 C (bioMérieux, France) (Figure 1) ( 8 ). All isolates were stored as suspensions in sterile distilled water at 4°C temperature until used in the study.
3.2. Suspension Preparation
All tested yeasts were sub cultured on Sabouraud dextrose broth (Merck, Germany) and incubated at an ambient temperature in an orbital shaker for 48 h aerobically. Cultures were centrifuged at 2000g for 10 min. Yeast sediments were washed with phosphate buffered saline (PBS) twice, and then adjusted to a concentration of 106 cells/mL.
3.3. Susceptibility of Isolates to Antifungal Agent
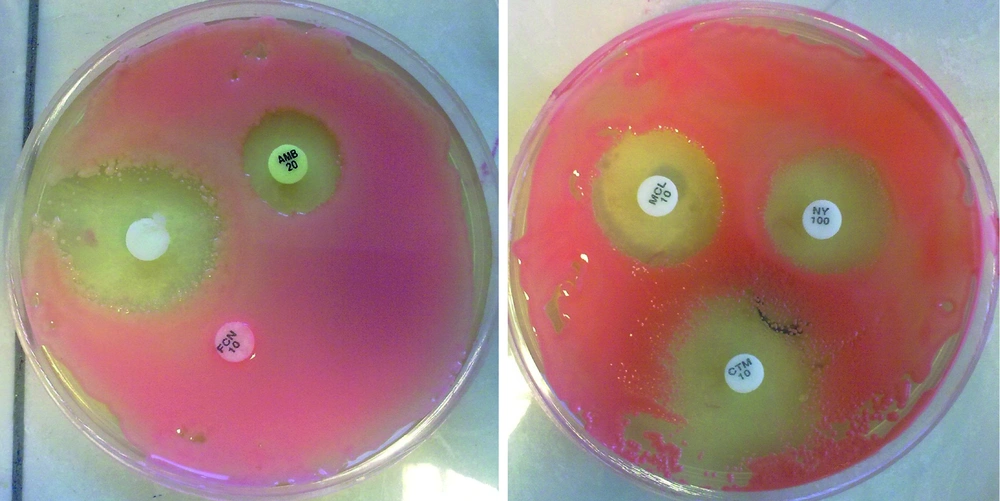
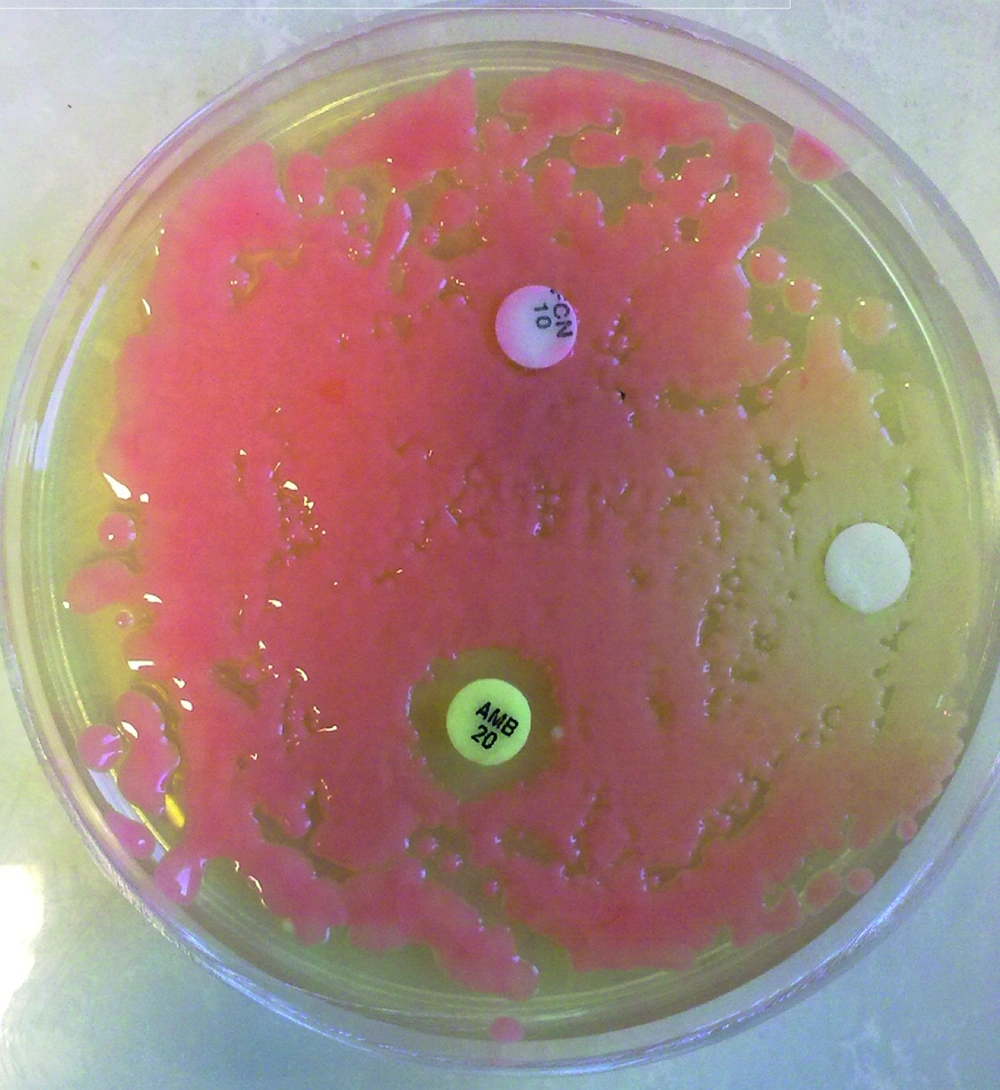
We studied a total of 69 different strains of Rhodotorula that were isolated from two hospitals in Ahvaz. Prior to testing, each isolate was sub-cultured at least twice on SDA to ensure purity and optimal growth. In vitro susceptibility testing was performed by the disc diffusion method. The antifungal agents used in the study were as follows: amphotericin B (20µg), fluconazole (10 µg), miconazole (10 µg), clotrimazole (10 µg) and nystatin (100U), (Liofilchem Bacteriology Products, Italy). Terbinafine disks were also prepared at 50μg/disk. A suspension equivalent to 0.5 McFarland was prepared from an overnight yeast culture. 100 µl of the suspension was inoculated on SDA medium and this was spread evenly on the surface medium. Discs containing antifungal agents were placed on the medium. The inhibition zone was evaluated after 24-48 hours manually (Figure 2). Criteria for susceptibility to used antifungal drugs are summarized in Table 2 ( 22 - 25 ).
| Antifungals | Zone Diameter, mm | ||
|---|---|---|---|
| Sensitive | Dose Dependent | Resistance | |
| Nystatin | ≥ 25 | 17 - 24 | 16 |
| Clotrimazole | ≥ 20 | 12 - 19 | ≤ 11 |
| Miconazole | ≥ 20 | 12 - 19 | ≤ 11 |
| Terbinafine | ≥ 20 | 12 - 19 | ≤ 11 |
| Amphotericin B | ≥ 15 | 10 - 14 | ≤ 9 |
| Fluconazole | 19 | 15 - 18 | 14 |
Criteria of Susceptibility and Resistance of Antifungal Disks
4. Results
4.1. Isolation and Identification Rhodotorula Species
Out of the 600 samples taken from the two educational hospitals, 39 (6.5%) cases yielded positive cultures for different species of Rhodotorula (Table 1). As shown, 25.6% of positive cultures were sampled from the floor, walls and windows of different areas of both hospital environments. Patient’s room furniture with 15.4%, and water taps and refrigerators with 12.8% were ranked at the second and third most common sites that were contaminated with Rhodotorula species. Most of the isolated yeasts were recovered from cardiology, nephrology and urology wards.
Our study shows that the most common contaminated samples were phones and mobile phones (1 of 4, 25%), followed by floor, walls and windows (10 of 43, 23.3%), recycle bins (2 of 14, 14.3) and refrigerators (5 of 36, 13.9%) (Table 1). In the present study 72 isolates of Rhodotorula species were recovered from different samples from two educational hospitals in Ahvaz. The most common species was R. glutinis (62, 86.1%), followed by R. mucilaginosa (5, 6.9%), R. minuta (3, 4.2%), and Rhodotorula species (2, 2.8%).
4.2. Antifungal Susceptibility
In the present study 69 isolates of Rhodotorula including; R. glutinis (59), R. mucilaginosa (5), R. minuta (3) and Rhodotorula species (2) were examined for susceptibility tests against three groups of antifungals, polyenes (Amphotericin B, nystatin), azoles (clotrimazole, miconazole, fluconazole) and allylamine (terbinafine). Resistance to Amphotericin B was found in 5.8% of isolates whereas 52.2% and 42.0% of isolates were dose dependent and sensitive to drug, respectively (Table 3). Most isolates were sensitive to nystatin (71.0%) and only 11 isolates (16.0%) showed resistance. In our study all isolates of R. mucilaginosa were resistant to nystatin. Our study showed that clotrimazole was the most effective antifungal agent against Rhodotorula strains. 94.2% of isolates were sensitive to clotrimazole, 2.9% were dose dependent and 2.9% were resistance. 66.7% of isolates were sensitive to miconazole, whereas 31.9% and 1.4% were dose dependent and resistant (Table 3). Fluconazole exhibited no activity in vitro against all strains of Rhodotorula . Resistance to terbinafine was found in 37.7% of isolates, whereas only 26.1% of the tested isolates were sensitive and the rest were dose dependent (Table 3).
| Susceptibility | R. glutinis | R. mucilaginosa | R. minuta | Rhodotorula Sp. | Total |
|---|---|---|---|---|---|
| Amphotericin B | |||||
| Resistant | 3(4.4%) | 0(0.0%) | 1(1.4%) | 0(0.0%) | 4(5.8%) |
| Dose dependent | 31(44.9%) | 4(5.8%) | 1(1.4%) | 0(0.0%) | 36(52.2%) |
| Sensitive | 25(36.2%) | 1(1.4%) | 1(1.4%) | 2(2.9%) | 29(42.0%) |
| Total | 59(85.5%) | 5(7.2%) | 3(4.4%) | 2(2.9%) | 69(100%) |
| Nystatin | |||||
| Resistant | 4(5.8%) | 5(7.2%) | 2(2.9%) | 0(0.0%) | 11(16.0%) |
| Dose dependent | 8 (11.6%) | 0 (0.0%) | 1 (1.4%) | 0 (0.0%) | 9 (13.0%) |
| Sensitive | 47 (68.1%) | 0 (0.0%) | 0(0.0%) | 2 (2.9%) | 49 (71.0%) |
| Total | 59 (85.5%) | 5 (7.2%) | 3(4.4%) | 2 (2.9%) | 69 (100%) |
| Clotrimazole | |||||
| Resistant | 2 (2.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (2.9%) |
| Dose dependent | 1 (1.4%) | 0 (0.0%) | 1 (1.4%) | 0 (0.0%) | 2 (2.9%) |
| Sensitive | 56 (81.2%) | 5 (7.2%) | 2 (2.9%) | 2 (2.9%) | 65 (94.2%) |
| Total | 59 (85.5%) | 5 (7.2%) | 3(4.4%) | 2 (2.9%) | 69 (100%) |
| Miconazole | |||||
| Resistant | 0 (0.0%) | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 1 (1.4%) |
| Dose dependent | 19 (27.6%) | 2 (2.9%) | 0 (0.0%) | 1 (1.4%) | 22 (31.9%) |
| Sensitive | 40 (58.0%) | 2 (2.9%) | 3 (4.4%) | 1(1.4%) | 46(66.7%) |
| Total | 59 (85.5%) | 5 (7.2%) | 3 (4.4%) | 2 (2.9%) | 69 (100%) |
| Terbinafine | |||||
| Resistant | 21 (30.4%) | 4 (5.8%) | 0 (0.0%) | 1 (1.4%) | 26 (37.7%) |
| Dose dependent | 24 (34.8%) | 0 (0.0%) | 0 (0.0%) | 1 (1.4%) | 25 (36.2%) |
| Sensitive | 14 (20.3%) | 1 (1.4%) | 3 (4.4%) | 0 (0.0%) | 18 (26.1%) |
| Total | 59 (85.5%) | 5 (7.2%) | 3 (4.4%) | 2 (2.9%) | 69 (100%) |
| Fluconazole | |||||
| Resistant | 59 (85.5%) | 5 (7.2%) | 3 (4.4%) | 2 (2.9%) | 69 (100%) |
| Dose dependent | 0 (0.0%) | 0(0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Sensitive | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Total | 59 (85.5%) | 5 (7.2%) | 3(4.4%) | 2 (2.9%) | 69 (100%) |
Susceptibility of Rhodotorula Strains to Amphotericin B, Clotrimazole, Miconazole, Nystatin, Fluconazole and Terbinafine
In our study terbinafine inhibited the red pigmentation in Rhodotorula strains during antifungal testing (Figure 3).
5. Discussion
In recent years, the incidence of opportunistic mycosis has increased, due to the rise of predisposing factors. Yeasts, especially Candida species, have an important role in opportunistic fungal infection (26). Rhodotorula strains are commensal yeasts and they appear to be less virulent than more common yeasts (Candida and Cryptococcus). In addition, several reports show that Rhodotorula species have emerged as opportunistic pathogens in immunecompromised patients, during the last three decades (27, 28). Diekema et al. believed that mortality due to Rhodotorula infection has increased to 15% (8). Rhodotorula species are opportunistic red yeasts that are frequently isolated from air, soil, water, milk and their products, environmental substrates, shower curtains, toothbrushes and hospital equipment (29-31). They have also been detected in cultures from skin, urine, stool, sputum, respiratory secretions, gastric washing, blood, vagina, and cerebrospinal fluid of hospitalized patients (32, 33). However there are a few reports that show the presence of Rhodotorula in hospital environments, patients room furniture and medical instruments.
In the present study 6.5% of samples were positive for Rhodotorula species. In addition, their diversity was also due to differences in sampled sites. Our study showed that the most contaminant sample sites were phones and mobile phones, (1 in 4, 25%) and floor, walls and windows (10 in 43, 23.3%). Airborne mycobiota have been implicated in from allergies to disseminated fungal infections. Nosocomial fungal infections have become particularly important during the last three decades. Infection due to Rhodotorula strains is one of the most important nosocomial infections, and the presence of this organism in hospitals could be considered as a risk factor for hospitalized patients. Rhodotorula is increasingly being detected as a human pathogen during the last 2-3 decades(9, 12, 13, 15, 16, 27, 30).
In our study, most Rhodotorula strains were recovered from the cardiology, nephrology and urology wards. Patients with central venous catheters, urinary catheters and haematological patients usually stay for long durations in such wards. As a result, these patients are at risk of being contaminated by this organism. Biological contamination of hospital environments, medical instruments, patients rooms, protective, and critical and intensive care units may pose a potential health risk to patients (34). Based on the “ARTEMIS Global Antifungal Surveillance Program” Rhodotorula species are the fourth most common non-candidal yeasts isolated from clinical specimens (19).
Studies have shown that the distribution of fungi in the environment varies among geographic areas, and its distribution is affected by several factors; such as temperature, humidity, time of day and human activities (35). In a study conducted by Cordeiro et al. in two tertiary hospitals of Fortaleza, 23.8% of isolated fungi were Rhodotorula (26). However they did not detect the type of Rhodotorula. Our study demonstrates the occurrence of several species of Rhodotorula in different sites of two educational hospitals in Ahvaz. Cardiology, nephrology and urology wards were respectively the most contaminated sites. Our study showed that most of the isolated red strains of yeast-like fungi were R. glutinis followed by followed by R. mucilaginosa, and R. minuta. In a review on 59 cases of blood stream infection by Lunardi et al. R. mucilaginosa was the most common agent (18). However, R. glutinis was the second most recovered yeast from solid wastes and dental health service environments (21).
Zaas et al. were determined about the antifungal susceptibilities of 10 Rhodotorula bloodstream infection strains. They showed that all isolates were most susceptible to amphotericin B and flucytosine and less susceptible to azoles (12). In another study conducted by Gomez-Lopez et al. fluconazole, itraconazole and voriconazole were inactive in vitro against the majority of tested Rhodotorula strains. However, both amphotericin B and flucytosine exhibited good activity against all 29 tested isolates (17). Galan-Sanchez et al. tested 35 strains of Rhodotorula isolated from clinical material against several antifungal agents (36). They found that all the tested strains were sensitive to 5-fluorocytosine, amphotericin B, ketoconazole and itraconazole and resistant to fluconazole. 95% of our Rhodotorula were sensitive to amphotericin B. Our results confirm previous studies that had shown that fluconazole is inactive against Rhodotorula (8, 18, 36). There are no previous studies regarding the effect of clotrimazole, nystatin and miconazole on Rhodotorula for comparison. Our study showed that resistance to clotrimazole and miconazole was only found in one and two strains, respectively. However the frequency of resistance to nystatin was 16%.
Rhodotorula species are widely distributed in hospitals and could be critical as nosocomial fungal infections. There are no previous data regarding the susceptibility of Rhodotorula to terbinafine. In the present study 37.7% of the tested Rhodotorula strains were resistant to terbinafine. Interestingly terbinafine inhibited the producing red pigment in Rhodotorula without affecting its growth. In conclusion, we can state that all antifungal agents tested, except fluconazole, are useful medicaments for the treatment of infections by the Rhodotorula genus.